Table of Contents
- What is Formaldehyde?
- Formaldehyde Structure – CH2O
- Physical Properties of Formaldehyde – CH2O
- Chemical Properties of Formaldehyde – CH2O
- Uses of Formaldehyde – CH2O
- Frequently Asked Questions
What is Formaldehyde?
Formaldehyde is the simplest aldehyde made of hydrogen, carbon and oxygen with the formula CH2O.
Formaldehyde is one of a large family of chemicals known as volatile organic compounds which evaporates and becomes gaseous at room temperature.
Formaldehyde is a reactive molecule and the first in the series of aliphatic aldehydes. It is one of the most important industrial chemicals. Formaldehyde is usually described as a gas, but it also exists dissolved in water or other solvents. Formaldehyde is a naturally occurring substance used in a wide variety of applications.
Other names – formalin, methanal, formol
| CH2O | Formaldehyde |
| Density | 815 kg/m³ |
| Molecular Weight/ Molar Mass | 30.031 g/mol |
| Boiling Point | -19 °C |
| Melting Point | -92.0 oC |
| Chemical Formula | HCHO |
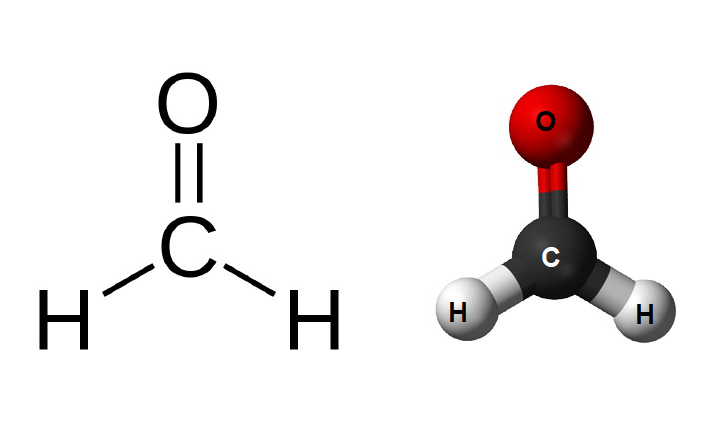
Formaldehyde Structure – CH2O
Physical Properties of Formaldehyde – CH2O
| Odour | Suffocating odour |
| Appearance | Colourless gas |
| Covalently-Bonded Unit | 1 |
| Hydrogen Bond Acceptor | 1 |
| Complexity | 2 |
| Solubility | Soluble in water and acetone. |
Chemical Properties of Formaldehyde – CH2O
-
- Formaldehyde reacts with a base like sodium hydroxide and forms sodium formate and methanol. The chemical equation is given below.
2HCHO + NaOH → HCOONa + CH3OH
-
- Formaldehyde reacts with ammonia to form formamidine and water. The chemical equation is given below.
6HCHO + 4NH3 → (CH2)6N4 + 6H2O
Uses of Formaldehyde – CH2O
- Used in the production of resins, principally urea-formaldehyde and phenol-formaldehyde, which are used to make cores and moulds for foundries.
- Used in agriculture and medicine where it is used as a disinfectant, fungicide, fumigant and preservative.
- Formaldehyde is used to produce a wide array of products, particularly building materials.
Frequently Asked Questions
What is formaldehyde made from?
Formaldehyde (HCHO), also known as methanal, is an organic compound, the simplest of the aldehydes, used in large quantities in a number of processes of chemical processing. It is formed primarily by methanol vapour-phase oxidation and is commonly sold as formalin, an aqueous solution of 37 percent.
Why is formaldehyde a good preservative?
Formaldehyde is a combination of hydrogen, oxygen, and carbon. Because of its antibacterial properties, it is widely used as a preservative.
Why is formaldehyde used in vaccines?
Formaldehyde has a long history of safe use in producing some bacterial and viral vaccines. This is used to inactivate viruses to avoid disease, such as poliovirus used to produce polio vaccines and to detoxify bacterial toxins, such as the toxin used to create diphtheria vaccines.
How is formic acid converted to formaldehyde?
Upon the addition of hydrogen, the formic acid is converted to formaldehyde. So formaldehyde is more easily oxidised into formic acid by ambient oxygen. Formic acid is miscible with water, and most organic solvents with polarity.
How do you neutralise formic acid?
Baking soda (NaHCO3) is also used for the neutralization of acids, including formic acid. You would want to neutralise it with a thick paste of sodium bicarbonate water (NaHCO3) if you were to spill some formic acid solution on your skin or on the concrete.


Comments