What is Urea?
Urea is a nitrogenous compound formed in the liver. It has a chemical formula of CH4N2O. It is also known as Carbamide or Ureophil. This compound is the final end product of protein metabolism. It is a waste product and has no physiological function. It dissolves in blood and kidney excretes it in urine. This organic compound has two NH2 groups connected by a functional group carbonyl. Urea dissolves in water and is non-toxic. It is colourless and has no smell. It is widely used as an important raw material in industries and commonly used in fertilizers.
Table of Contents
Properties of Urea – CH4N2O
| CH4N2O | Urea |
| Molecular Weight/ Molar Mass | 60.06 g/mol |
| Density | 1.32 g/cm3 |
| Appears | White solid |
| Melting Point | 133 °C |
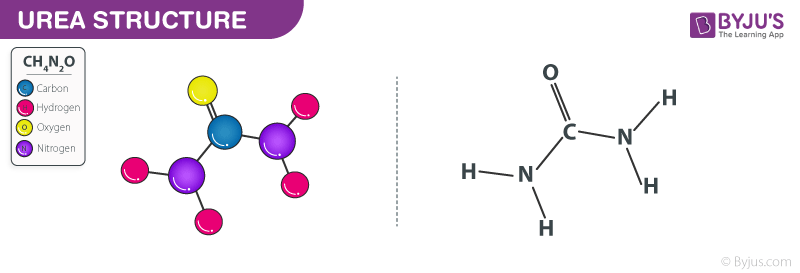
Urea structure – CH4N2O
CH4N2O Uses (Urea)
- It is used as a nitrogen-release fertilizer
- It is used as a stabilizer in nitrocellulose explosives
- It is used in lanthanide chemistry as an important reagent
- It is used in the manufacturing of high explosive like urea nitrate
- It is used in creams or ointments to rehydrate skin
- It is used in the urea breath test to detect the presence of bacteria in the stomach
- It is used as an ingredient in dish soap
- It is used in hair removal creams
- It is used in making pretzels as a browning agent
- It is used in the manufacture of melamine
Frequently Asked Questions
Are urea and urine the same?
Urea (commonly referred to as carbamide) is a waste product that is produced in most living organisms. This compound is human urine’s main organic component. The liver transforms the ammonia into a non-toxic compound, urea, which can then be transferred safely into the kidneys in the blood, from where it is excreted in urine
What are the uses of urea?
The diamide of carbonic acid, Urea, is also called carbamide. The chemical formula of this compound is H2NCONH2. Urea has essential uses as a supplement to fertilizer and feed, as well as starting material for plastics and medicines manufacturing.
How does urea affect the skin?
The nitrogen compound urea has beneficial water-binding and mild exfoliating properties for skin in limited quantities. It can cause irritation in larger quantities, but it can also exfoliate skin in large amounts. Urea may make other cosmetic ingredients more absorbent on the skin.
Also Read:
| Ammonia | Nitrous oxide |
| Cyanide | Carbon monoxide |
Learn more about the properties, production and the structure of CH4N2O from the expert faculties at BYJU’S.

Comments