What is Potassium Ferrocyanide?
“Potassium ferrocyanide is an inorganic compound with the chemical formula K4Fe(CN)6. Potassium ferrocyanide is also known as yellow potash prussiate, a yellow crystal.”
It was made with either wool or horn clippings stirring hot potassium carbonate with an iron rod.
Other names – yellow prussiate of potash, potassium hexacyanoferrate (II)
| K4Fe(CN)6 | Potassium ferrocyanide |
| Density | 1.85 g/cm³ |
| Molecular Weight/ Molar Mass | 368.35 g/mol |
| Boiling Point | 400 °C |
| Melting Point | 300 °C |
| Chemical Formula | K4Fe(CN)6 |
Table of Contents
- Potassium Ferrocyanide Structure – K4Fe(CN)6
- Physical Properties of Potassium Ferrocyanide – K4Fe(CN)6
- Chemical Properties of Potassium Ferrocyanide – K4Fe(CN)6
- Uses of Potassium Ferrocyanide – K4Fe(CN)6
- Frequently Asked Questions
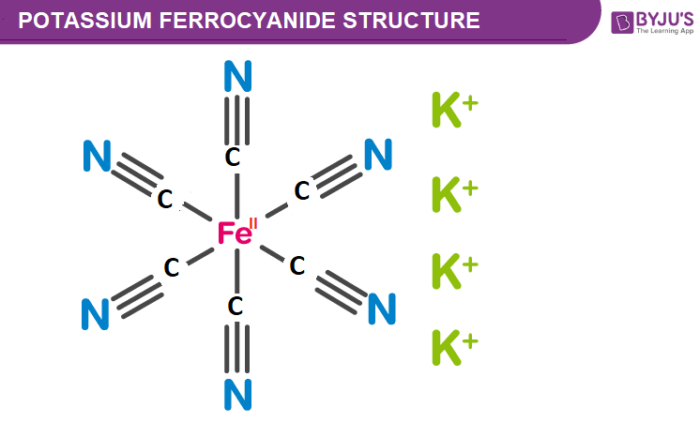
Potassium Ferrocyanide Structure – K4Fe(CN)6
Physical Properties of Potassium Ferrocyanide – K4Fe(CN)6
| Odour | No odour |
| Appearance | Light yellow, crystalline granules |
| Covalently-Bonded Unit | 10 |
| Hydrogen Bond Acceptor | 12 |
| Complexity | 127 |
| Solubility | Insoluble in ethanol, ether |
Chemical Properties of Potassium Ferrocyanide – K4Fe(CN)6
- The reaction of potassium ferrocyanide reacts with sulphuric acid in an aqueous medium and forms potassium sulphate, ferrous sulphate, ammonium sulphate and carbon monoxide. The chemical equation for the same is shown as:
K4[Fe(CN)6] + 6H2SO4 + 6H2O → 2K2SO4 + FeSO4 + 3(NH4)2SO4 + 6CO
- Potassium ferrocyanide reacts with ferric chloride to form a complex compound Iron (III) potassium hexacyanidoferrate(II) and potassium chloride.
K4[Fe(CN)6] + FeCl3 → KFe[Fe(CN)6] + 3 KCl
Uses of Potassium Ferrocyanide – K4Fe(CN)6
- Used in the tempering of steel and in process engraving. It is employed in the manufacture of pigments and as a chemical reagent.
- A small amount of pyro and hydroquinone developers tends to lower fog and give greater density.
- Used in the manufacture of potassium cyanide, which is used extensively in gold mining.
- Ferro Cyanogen forms with most metal compounds insoluble in water, some of which exhibit highly characteristic colours. It serves as a test for cupric and ferric compounds.
- Potassium ferrocyanide was used for certain iron processes as a developer and as an additive for developers of alkaline pyro

Comments