Table of Contents
- What is Water?
- States:
- Physical and Chemical Properties of Water:
- The Importance of Water:
- Recommended Video
What is Water?
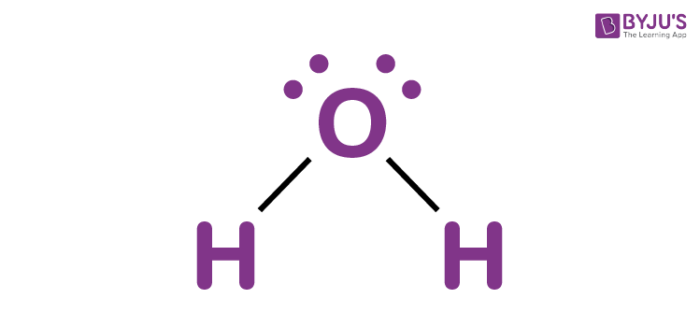
Water is a colourless and transparent chemical substance which is the main constituent of streams, oceans, and lakes on the earth’s crust. It is an important fluid that plays a significant role in sustaining life on earth. The chemical formula of water is H2O. It is made up of two hydrogen atoms and one oxygen atom which are held together by covalent bonds. 71% of the earth’s surface is made up of this liquid.
States:
- The solid state of water is known as ice. Water freezes at 0o Celsius (freezing point of water) to form ice.
- The liquid state of water makes up most of the earth’s surface. It finds its application in a wide range of areas.
- The gaseous state of water is known as water vapour. At 100o Celsius water reaches its boiling point and gets converted into water vapour.
Physical and Chemical Properties of Water:
- It is a colourless and tasteless liquid.
- Compared to other liquids, it has higher thermal conductivity, specific heat, surface tension, etc.
- Water is referred to as a universal solvent as most substances such as acids, salts, sugar, and gases readily dissolve in it.
- Water has the capability to form azeotropes with other solvents.
- The electrical conductivity of water is low. However, it can be improved by adding ionic substances that dissociate to give ions in the aqueous phase for carrying the charge.
Know more about the properties of water.
The Importance of Water:
-
Agriculture:
The most important use of water is for agriculture. Irrigation is necessary for agriculture, and for that, water is the key component to produce food.
-
Drinking:
The human body contains 50% to 78% of water according to the size of the body. Humans need to drink 7 litres of water every day to avoid dehydration.
-
Health:
It plays an important role in digestion and other biological processes that occur in living organisms. It plays an important role in maintaining the pH of the body. It also helps in the movement of antibodies from the immune system.
-
Regulator:
It helps in regulating body temperature. Water provides the necessary cooling effect to the body.
-
Intoxication:
Water removes harmful toxins from the body through perspiration and urination. It prevents the building up of wastes in living organisms.
-
Washing:
It is used to make emulsions and solutions which are used for washing purposes.

To know about the water cycle and more topics, register with BYJU’S and download our app.
Recommended Video




Comments