Colloids are an important class of heterogeneous mixtures which are formed by mixing dispersed phase with the dispersion medium. Most substances like creams, milk, curd, and medicines are colloids. Colloids show special features like the Tyndall effect and the Brownian movement. In this article, there is a detailed discussion about the coagulation of colloids.
Table of contents
- Definition of coagulation of colloidal solution
- Coagulation techniques
- Hardy Schulze Law of coagulation
- Coagulation of lyophilic solutions
- Coagulation of lyophobic solutions
- FAQs
Definition of coagulation of colloidal solution
Coagulation is a process of aggregation or accumulation of colloidal particles to settle down as a precipitate.
Substances like metals, their sulfides etc cannot be simply mixed with the dispersion medium to form a colloidal solution. Some special methods are used to make their colloidal solutions. Such kinds of sols are known as lyophobic sols. These kinds of colloidal solutions always carry some charge on them. All the particles of a given colloidal system have like charges because of which they don’t combine together and settle down. This is the main cause of the stability of colloids. If by any chance we can remove the charge present on the sol, the particles get closer to each other, and they accumulate to form aggregates and precipitate under the action of gravity. This process of accumulation and settling down of particles is further known as coagulation or precipitation.
Coagulation Techniques:
The process of coagulation can be carried out in the following ways:
- By electrophoresis: In this method, the colloidal particles are forced to move towards the oppositely charged electrodes, and then they are discharged and collected at the bottom.

2. By mixing two oppositely charged sols: In this type of coagulation equal amounts of oppositely colloids are mixed which mutually coagulates their charges resulting in the precipitation.
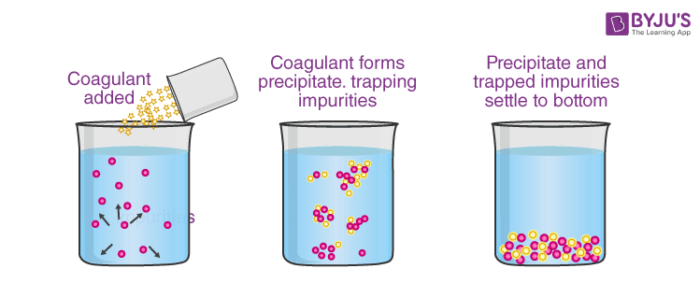
Fig. Coagulation by mixing two oppositely charged sols
3. By boiling: Whenever we boil a sol, the molecules of the dispersion medium start colliding with each other and with the surface, this, in turn, disturbs the adsorption layer. This reduces the charge on the sol due to which the colloid particles come together, form aggregates and settle down.
4. By persistent dialysis: Under persistent dialysis parts of electrolytes are removed completely and the sol loses its stability and ultimately coagulates.
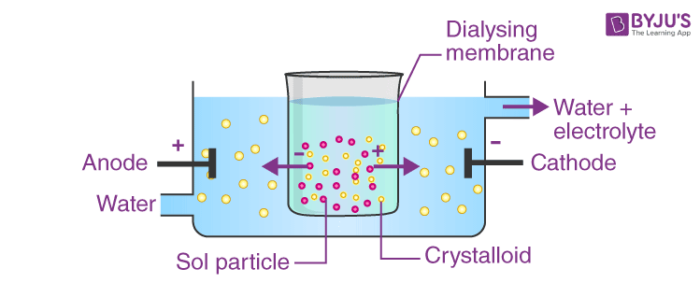
Fig. Coagulation by Persistent Dialysis
Hardy Schulze Law of coagulation:
According to Hardy Schulze law, the quantity of electrolyte required to coagulate a definite amount of colloidal solution depends upon the valency of the coagulating ion.
- The coagulating ions are the ions of electrolyte which carry the opposite charge of the colloidal particles.
- The greater the valency of the coagulating ion, the greater the coagulation power.
- The coagulation of a negatively charged sol (As2S3)is done by adding a positively charged colloid. The coagulating values of different electrolytes are given below:
| Electrolyte | Cation | Coagulating value |
| NaCl | Na+ | 52 |
| MgCl2 | Mg2+ | 0.72 |
| BaCl2 | Ba2+ | 0.69 |
| AlCl3 | Al3+ | 0.093 |
Coagulation ∝ 1/ Coagulating value (Coagulation is inversely proportional to coagulating value)
So the order of coagulating power of cations for negatively charged sol As2S3.
Al3+ > Ba2+ > Mg2+ > Na+
- For coagulation of a positively charged sol (Fe(OH)3) the coagulating ion is an anion, the power of coagulating value are given below :
| Electrolyte | Anion | Coagulating value |
| KBr | Br – | 138 |
| K2SO4 | SO42- | 0.210 |
| K2C2O4 | C2O42- | 0.238 |
| K3[Fe(CN)6] | [Fe(CN)6]3- | 0.096 |
Coagulation ∝ 1/ Coagulating value
So the order of coagulating power of anion for positively charged sol Fe(OH)3.
Br– < SO42- < [Fe(CN)6]3-
Coagulation of lyophilic solutions:
Stability of lyophilic sol depends on the following two factors
- Charge on the colloidal particles
- Solvation of the colloidal particles
When the charge and solation layers are disturbed or removed, are removed then only lyophilic sols can be coagulated. This can be done by either adding an electrolyte or a suitable solvent.
Coagulation of lyophobic solutions:
Lyophobic sols are less stable than lyophilic colloid. Hence they are more easily coagulated.
The stability of lyophobic sol is only due to the charge on the colloidal particles. This factor can be removed by adding only electrolytes.
This was just a brief layout of the coagulation of lyophilic and lyophobic colloids. To know more about the process of coagulation, you can register with BYJU’S and download our app.
Frequently Asked Questions- FAQs
What causes coagulation in a colloidal solution?
The charge present on the colloidal sols determines their stability. If we can remove the charge present on the sol, the particles get closer to each other, and they accumulate to form aggregates and precipitate under the action of gravity. So the electrolyte is added to the sol to neutralize the charge and settle down particles that cause coagulation or precipitation.
What is the coagulation value?
The amount of electrolytes added to 1 litre of a colloidal solution to form coagulation or precipitation is called coagulation value.
What is the Hardy Schulze rule?
The quantity of electrolyte required to coagulate a definite amount of colloidal solution depends upon the valency of the coagulating ion is known as the Hardy Schulze rule.
What modification can you suggest to the Hardy Schulze rule?
Hardy Schulze’s rule should be modified on the basis of the polarizing power of the flocculating ion causing precipitation. Hence the modified law will be as the greater is the power of flocculating ion added, the higher will be its power to cause precipitation.
What is a coagulating ion?
Coagulating ion is an ion in the electrolyte which neutralises the opposite charge on colloid and coagulates it.

Very good explanation for coagulation in chemistry
Thank you byjus