What is Electron Dot Structure?
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known.
It defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
The representation of molecules in Lewis electron dot structure or just a Lewis structure is in honour of the American chemist Gilbert Newton Lewis.
Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor.
Table of Contents
Lewis Dot Structure
Lewis dot structures reflect the electronic structures of the elements, including how the electrons are paired. Lewis structures are a useful way to summarize certain information about bonding and may be thought of as “electron bookkeeping”. In Lewis dot structures each dot represents an electron. A pair of dots between chemical symbols for atoms represents a bond.
Recommended Videos

How to Draw Electron Dot Structures?
A Lewis Electron Dot Formula comprises one dot for every valence electron and an element’s symbol. Stages to articulate the electron dot formula are stated beneath. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols.
Pick up every valence electrons from every atom and toss them into a make-believe container that we can term as electron pot.
Make use of the N – A = S equation to get to know the number of bonds
-
-
-
-
-
- The sum of the number of valence electrons is equal to N
- The number of valence electrons in electron pot is A.
- The number of electrons shared in the molecule equals S
- Distribute the electrons from electron pot to account for the bonds
- Distribute the rest of the electrons
-
-
-
-
How to Draw Lewis Structures?
A Lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms.
The steps that must be followed while drawing a Lewis structure are listed below.
-
-
-
-
-
- First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom.
- If the molecule is an anion, extra electrons (number of electrons added = the magnitude of negative charge) are added to the Lewis dot structure.
- When cationic molecules are considered, electrons are subtracted from the total count in order to make up for the positive charge.
- The least electronegative atom is made the central atom of the molecule or ion.
- The atoms are now connected via single bonds.
- Now, the lone pairs of electrons are assigned to each atom belonging to the molecule. Commonly, the lone pairs are assigned to the most electronegative atoms first.
- Once the lone pairs are assigned, if every atom does not have an octet configuration, a double or triple bond must be drawn to satisfy the octet valency of each atom.
- If required, a lone pair can be converted into a bond pair in order to satisfy the octet rule for two atoms.
-
-
-
-
It is important to note that only the valence electrons are considered while drawing Lewis dot structures and the electrons that do not belong to the outermost shell are ignored.
Lewis Structure Examples
The Lewis electron dot structures of a few molecules are illustrated in this subsection.
1. Lewis Structure of CO2
-
-
-
-
-
- The central atom of this molecule is carbon.
- Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
- Carbon contains four valence electrons, resulting in zero lone pairs. Therefore, it is doubly bonded to each oxygen atom.
-
-
-
-
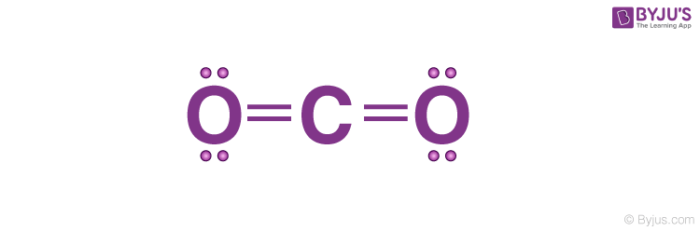
2. Lewis Structure of O2
-
-
-
-
-
- An atom of oxygen contains 6 electrons in the valence shell.
- Four of the valence electrons exist in lone pairs, implying that the oxygen atom must participate in two single bonds or one double bond in order to attain an octet configuration.
- Since there are only two oxygen atoms in an O2 molecule, the atoms form a double bond resulting in the following Lewis electron dot structure.
-
-
-
-

3. Lewis Structure of CO (Carbon Monoxide)
-
-
-
-
-
- A carbon monoxide molecule consists of one carbon atom and one oxygen atom.
- The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two.
- Therefore, the valency is satisfied via the donation of a lone pair of electrons for bonding by the oxygen atom.
- The resulting Lewis electron dot structure displays a triple bond connecting a carbon and an oxygen atom, each holding a lone pair of electrons.
-
-
-
-

Solved Examples
Problem-1: In terms of electron dot formulas, define the electron structure of the carbonate ion CO32-.
Solution:
One potential electron dot formula for the carbonate ion is

Since all carbon-oxygen bonds are likely to be equal, the electron structure in resonance terms is shown below.
Over the region of all three carbon-oxygen bonds, one electron pair is delocalized.
Problem-2: Cyclopentane – What will be the formula and electron dot structure of this element?
Solution:
Single bonds connect five carbon atoms of cyclopentane in a cyclic form. The molecular formula of cyclopentane is C5H10. The electron dot structure and structural formula of cyclopentane are articulated underneath.

Thus, the concepts related to Lewis dot structures are discussed briefly in this article along with a few examples.
Frequently Asked Questions – FAQs
What electrons do Lewis structures show?
The outermost central level of energy-containing electrons is called the level of valence and includes electrons of valence. Lewis symbols are diagrams showing the number of valence electrons of a specific element with dots indicating lone pairs.
How do electron dot structures represent shared electrons?
Electron dot structure-valence electrons are represented by dots around the symbol of elements. Electrons sharing — covalent bonding — electrons sharing is the glue that holds atoms together.
What is the purpose of Lewis structures?
The aim of Lewis structures is to provide a simple way for chemists to view molecules that allows accurate predictions about the actual molecules and structure and properties to be made.
What is the Lewis dot structure in chemistry?
Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule’s atoms and the lone pairs of electrons that may occur in the molecule.
What is the Lewis structure of ammonia?
Ammonia has the molecular formula NH3. It is extremely water-soluble because it is a polar material. For a molecule, the Lewis structure is the total valence electrons in the molecule. In ammonia, the nitrogen atom has eight valence electrons.
Why are Lewis structures important?
For the prediction of geometry, polarity and reactivity of (in)organic compounds, Lewis structures are actually very important. The Lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom.
To learn more about this topic and other related topics, register with BYJU’S and download the mobile application on your smartphone.


Comments