What is Silver Nitrate?
Silver nitrate is a chemical compound with the formula AgNO3. It consists of an ionic bond between the silver cation (Ag+) and the nitrate anion (NO3–). Due to the ionic nature of this compound, it readily dissolves in water and dissociates into its constituent ions.
Silver nitrate is a precursor to many compounds of silver, including the silver compounds used in photography. When compared to silver halides, which are used in photography due to their sensitivity to light, AgNO3 is quite stable when exposed to light.
Table of Contents
- Structure of AgNO3
- Properties of Silver Nitrate
- Physical Properties
- Chemical Properties
- Uses of Silver Nitrate
- Frequently Asked Questions
Structure of AgNO3
An illustration describing the structure of the silver nitrate molecule is provided below. It can be observed that silver has an oxidation number of +1 in this compound.
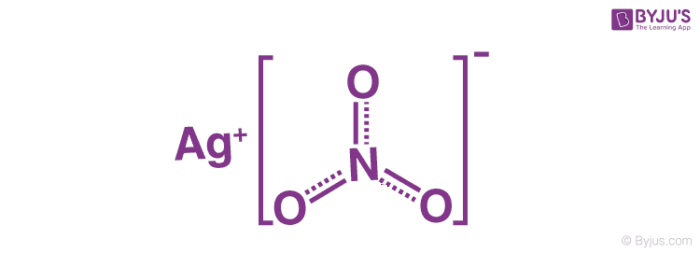
The nitrate ion described above consists of one nitrogen atom which is surrounded by three oxygen atoms. The nitrogen-oxygen bonds in this ion are similar to each other. The formal charge assigned to the nitrogen atom is -1, whereas each oxygen atom holds a charge of -⅔. The net charge associated with the nitrate ion is -1, which is quenched by the +1 charge held by the Ag+ ion via an ionic bond in AgNO3. It can be noted that the structure of the nitrate ion is stabilized by resonance.
Properties of Silver Nitrate
Some important physical and chemical properties of silver nitrate are listed in this subsection.
Physical Properties
- The molar mass of silver nitrate is 169.872 grams per mole.
- AgNO3 has a colourless appearance in its solid-state and is odourless.
- In its solid state, it has a density of 4.35 grams per cubic centimetre. Its density in the liquid state at a temperature of 210 oC corresponds to 3.97 g/cm3.
- The melting and boiling points of silver nitrate are 482.8 K and 713 K respectively. However, this compound tends to decompose at temperatures approaching its boiling point.
- Silver nitrate, like most ionic compounds, dissolves readily in water. Its solubility in water corresponds to 122 g /100mL at 0 oC and 256g / 100mL at a temperature of 25 o
- The crystal structure of AgNO3 is orthorhombic.
Chemical Properties
- The hazards of AgNO3 include its toxic and corrosive nature.
- The reaction between silver nitrate and ethanol is explosive.
- The silver present in the silver nitrate compound is displaced by copper, which forms copper nitrate. The chemical equation for this reaction is given by 2AgNO3 + Cu → Cu(NO3)2 + 2Ag
- When heated to 440 oC, this compound completely decomposes to give oxygen, nitrogen dioxide, and silver.
- Silver nitrate on decomposition gives silver, oxygen gas and nitrite. The chemical equation for the same is shown as:
\2AgNO_3(l)\rightarrow 2Ag(s) + O_2(g) + 2NO_2(g)\
It can be noted that even though metal nitrates generally decompose to yield metal oxides, the decomposition reaction of silver nitrate gives rise to elemental silver because silver oxide decomposes at an even lower temperature than AgNO3.
Uses of Silver Nitrate
Silver nitrate has a wide range of applications in many fields such as biology, chemical synthesis, and medicine. Some of these uses of AgNO3 are listed below.
- Silver nitrate is a very versatile compound because the nitrate ion can be replaced by other ligands that can bind to the silver ion.
- Due to the ability of this compound to form a precipitate of silver halides when treated with halide ions, it is used while making photographic films.
- Many silver-based explosives can be prepared with a precipitation reaction of silver nitrate.
- In the field of inorganic chemistry, halides are extracted with the help of this compound.
- The branch of chemistry known as analytical chemistry uses this reaction to check for the presence of halide anions such as the iodide, bromide, or chloride ions.
- Mixtures of alkenes can be separated with the help of this compound since the silver cation binds with alkenes in a reversible fashion.
- When diluted with water to a concentration of 0.5%, silver nitrate can serve as an antiseptic in many medical setups.
- A diluted solution of AgNO3 can be administered to the eyes of a baby which is born to a mother suffering from gonorrhea, which combats the gonorrhoea bacteria and protects the baby from the onset of blindness.
- This compound is also known to be used for the treatment and the removal of unwanted warts in human beings.
Frequently Asked Questions
What are the uses of silver nitrate?
Silver nitrate is widely used in many organic synthesis reactions in several ways. For example, for the deprotection and oxidation reactions. The Ag+ ion reversibly binds alkenes, and selectively adsorbing silver nitrate can be used to isolate alkene mixtures. The resulting adduct can be decomposed (in order to release the free alkene) with ammonia. Silver nitrate has been, in the past, used for silver staining (a process that employs silver or silver compounds to selectively change the appearance of a specific object). This compound is also used in medicine owing to its antiseptic qualities.
Is silver nitrate dangerous?
Silver nitrate is an oxidant and must, therefore, be kept away from organic compounds. Despite its widespread usage (especially in extremely low amounts) for the prevention of gonorrhoea and to stop bleeding from the nose, silver nitrate is often highly toxic and corrosive. Short-term exposure to this compound does not cause any immediate side effects apart from the development of a violet, brown or black stain on the part of the skin that was in contact with the silver nitrate. However, exposure to this compound over long periods of time is often accompanied by damage to the eyes. This compound is widely classified as an irritant to the skin and the eyes.
How is silver nitrate prepared?
Silver nitrate is usually prepared by combining silver with nitric acid. Common silver objects used in these reactions include silver bullions and silver foils. The products formed in this reaction include silver nitrate, water, and nitrogen oxides. The by-products of this chemical reaction depend on the nitric acid concentration that is used. It is important to note that this reaction must be carried out under a fume hood because of the evolution of poisonous oxides of nitrogen during the chemical reaction.
![]()
Thus, the important physical and chemical properties of silver nitrate are briefly described along with its properties. To learn more about this compound and other silver compounds such as silver chloride, register with BYJU’S and download the mobile application on your smartphone.

Is silver nitrate a colloid.
Colloidal silver is produced when gelatine-containing, aqueous silver solutions absorb ionizing radiation. In the presence of organic solutes, decrease is increased.