What is Matter in Chemistry?
As discovered by scientists,
The matter is made up of very tiny particles and these particles are so small that we cannot see them with naked eyes.
It has been observed that matter exists in nature in different forms. Some substances are rigid and have a fixed shape like wood and stone; some substances can flow and take the shape of their container like water, while there are forms of matter that do not have definite shape or size such as air.
Table of Contents
- Matter Definition
- Recommended Videos
- Solid Definition
- Liquid Definition
- Gas Definition
- Plasma
- Bose-Einstein Condensates
- Frequently Asked Questions – FAQs
Matter can be classified into different categories based on the physical properties exhibited by them and the states in which they exist; these are called states of matter.
Following are the basic three states of matter:
- Solid
- Liquid
- Gas
Apart from the above mentioned three, there are 2 more states of matter which we do not see in our everyday life. They are Plasma & Bose-einstein condensate.
Changes in the characteristics of matter related with external influences such as pressure and temperature separate states of matter. A discontinuity in one of those qualities frequently distinguishes states: rising the temperature of ice, for example, generates a discontinuity at 0 °C (32 °F) as energy flows into a phase transition rather than temperature rise.
Matter Definition Chemistry
Chemistry is the study of the composition of matter and its transformation. Another term often considered synonymous with matter is substance, but a substance has a more limited definition in chemistry. Chemistry deals with the study of behaviour of – matter Chemistry is concerned with the – Composition, structure and properties of matter and the phenomenon which occurs when different kinds of matter undergo changes.
Matter theory covers the changing ideas and systems that were used to describe and explain the material world. A large part of matter theory was based on a theory of the elements.
Recommended Videos
Matter In Our Surroundings – States of Matter

Solid Definition
- In solids, particles are tightly or closely packed.
- The gaps between the particles are tiny and hence it is tough to compress them.
- Solid has a fixed shape and volume.
- Due to its rigid nature, particles in solid can only vibrate about their mean position and cannot move.
- Force of attraction between particles is adamant.
- The rate of diffusion in solids is very low.
- An example of solids: solid ice, sugar, rock, wood, etc.
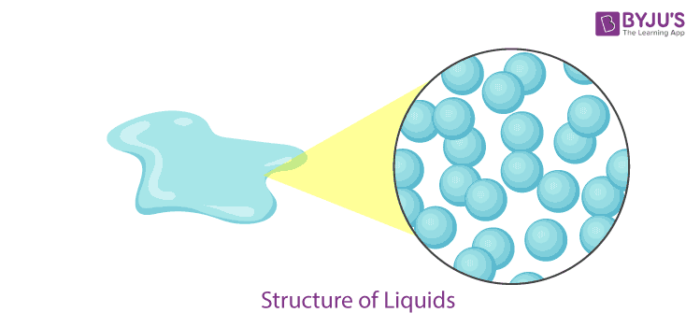
Liquid Definition
- In a liquid state of matter, particles are less tightly packed as compared to solids.
- Liquids take the shape of the container in which they are kept.
- Liquids are difficult to compress as particles have less space between them to move.
- Liquids have fixed volume but no fixed shape.
- The rate of diffusion in liquids is higher than that of solids.
- Force of attraction between the particles is weaker than solids.
- Example of a liquid state of matter: water, milk, blood, coffee, etc.
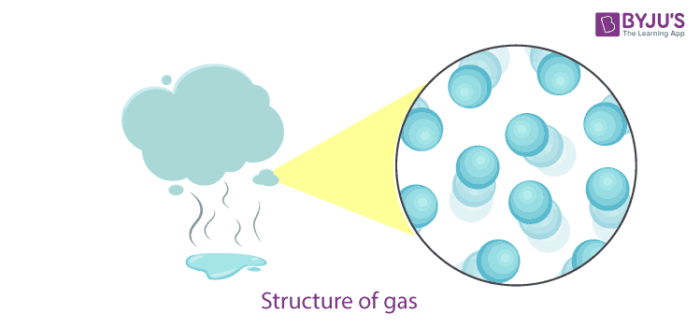
Gas Definition
- In gases, particles are far apart from each other.
- Force of attraction between the particles is negligible, and they can move freely.
- Gases have neither a fixed volume nor a fixed shape.
- The gaseous state has the highest compressibility as compared to solids and liquids.
- The rate is diffusion is higher than solids and liquids.
- The kinetic energy of particles is higher than in solids and liquids.
- An example of gases: air, helium, nitrogen, oxygen, carbon dioxide, etc.
Plasma
- Plasma is a not so generally seen form of matter. Plasma consists of particles with extremely high kinetic energy. Electricity is used to ionize noble gases and make glowing signs, which is essentially plasma.
- Superheated forms of plasma are what stars are.
Bose-Einstein Condensates
- Discovered in 1995, Bose-Einstein condensates were made with the help of the advancements in technology.
- Carl Weiman and Eric Cornell cooled a sample of rubidium with the help of magnets and lasers to within a few degrees of absolute zero.
- At the said temperature, the motion of the molecules becomes negligible. As this brings down the kinetic energy, the atoms no longer stay separate, but they begin to clump together. As the atoms join together they form a super-atom.
- Light slows down as it passes through a BEC helping scientists to study more about the nature of light as a wave and particle.
- BEC’s also show properties of a superfluid which implies, that it flows without friction.
Related Videos
Ideal Gas Equation & Its Applications

Kinetic Theory of Gases

Frequently Asked Questions – FAQs
What are the three common states of matter?
The common thing among the three states of matter is-they consist of tiny, small particles. They have a specific mass and can take up space. There is a volume in these three states. In these three states ‘atoms have the strength of attractions between them.
Can matter be created?
In addition, the first law of thermodynamics does not state that matter can not be created or destroyed, but rather that the total amount of energy in a closed system can not be created or destroyed, although it can be modified from one form to another.
Is matter created or destroyed?
There is a scientific law called the Mass Conservation Law, which Antoine Lavoisier discovered in 1785. It states in its most compact form: matter is not created or destroyed. The universe’s total mass and energy is constant.
Is matter-energy?
The mass of these three particles is less than a neutron’s mass, so each of them still gets some energy. So the same thing is really power and matter. Fully interchangeable. So in a way, all facets of the same thing are energy, matter, space and time.
What is Einstein’s theory of relativity?
In 1905, Albert Einstein determined that for all non-accelerating observers, the laws of physics were the same and that the speed of light in a vacuum was independent of all observers ‘ movement. This was the special relativity theory.
To know more about the states of matter, properties of matter and more, register with BYJU’S and download our app.
Read more:



It is very helpful 👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍👍
this vdieo is very nice and we will learn intersting
Thank you So Much it helped me very much in my school Project awesome information. Thank you very much The video is informative.
It is very good aap i can give a practice for it