What are Organic Compounds?
The compounds in solid, liquid or gaseous states which contain carbon in their molecule are known as organic compounds.
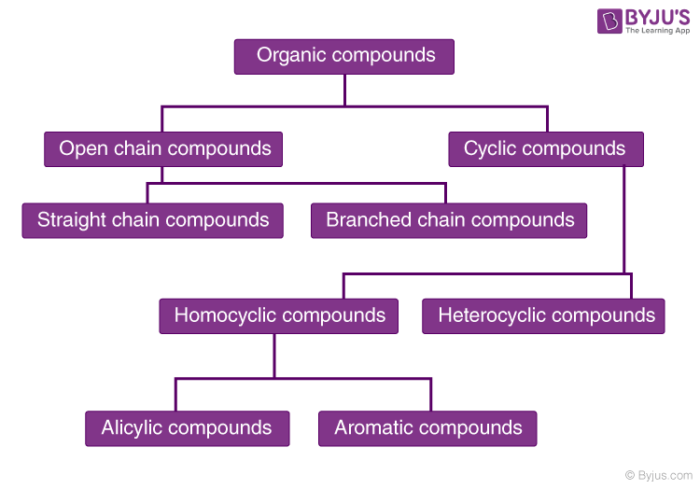
There are a large number of organic compounds and therefore a proper systematic classification was required. Organic compounds can be broadly classified as acyclic (open chain) or cyclic (closed chain).
Table of Contents
- Organic Compounds Classification
- Organic Compounds
- Recommended Videos
- What is an Organic Compound?
- Frequently Asked Questions – FAQs
Organic Compounds Classification
Moving on to their classification in detail:
Organic compounds are seen in a number of formats, including Lewis structures, space filled models and structural formulas. It is not uncommon to view the hydrogens as lines or to leave them all together in a structural formula of an organic molecule. They are understood to be present in order to complete the 4-bonds provided by the carbon atoms.
Organic compounds have been detected by mass spectra. The results obtained indicate that all extractants were able to perform direct analysis after extraction without any clean-up phase. Nevertheless, the form of organic compounds extracted depending on the solvent used, suggesting their distinct ability to solubilize various biosolid organic compounds.
Organic Compounds
Organic chemistry was once thought to be confined to the study of substances produced as part of the natural processes of living organisms, but as Friedrich Wohler discovered in the early 1800s, organic compounds can be synthesized from minerals and other non-organic materials in the laboratory. Indeed, modern chemistry and materials sciences have concentrated on the remarkable properties of carbon atoms for the production of synthetic chemicals, pesticides and a host of other things. Organic compounds contain carbon, almost always bonded to another carbon and/or hydrogen.
Sometimes, other elements, such as phosphorus, nitrogen and oxygen, are also bound to carbons. There are a few carbon compounds that are not considered organic molecules. These involve carbon dioxide, carbon monoxide, cyanates, cyanides and other carbon-containing ion compounds.
Alcohols include chemicals such as ethanol and isopropanol. They are used as antiseptics and ethanol is a staple of the beverage industry. Finally, carboxylic acids include a wide range of chemicals, including pharmaceuticals. Aspirin, one of the oldest commercial medicines, contains carboxylic acid.
While there are millions of organic compounds, there is a fairly simple classification scheme for these compounds and a method for naming even the most complex organic compounds. This unit will focus on helping you to identify the classification of organic compounds and to name only some of the most common of these compounds.
Recommended Videos
Carbon Compounds
Previous Year Paper Solutions – NEET

What is an Organic Compound?
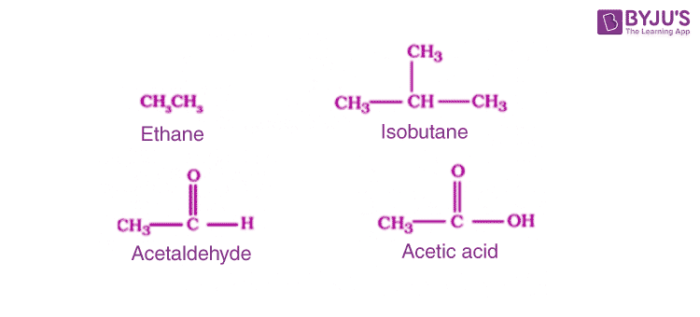
1. Acyclic or Open Chain Compounds:
These compounds are also known as aliphatic compounds, they have branched or straight chains. Following are the examples in this category.
2. Alicyclic or Closed Chain or Ring Compounds:
These are cyclic compounds which contain carbon atoms connected to each other in a ring (homocyclic). When atoms other than carbon are also present then it is called heterocyclic. Examples of this type are as follows:
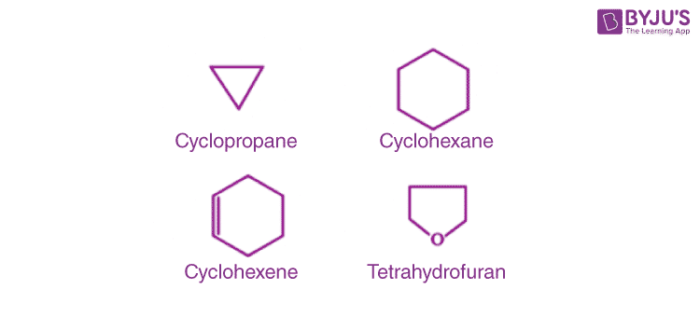
They exhibit some properties similar to aliphatic compounds.
3. Aromatic Compounds
They are a special type of compound which contains benzene and other ring related compounds. Similar to alicyclic, they can also have heteroatoms in the ring. Such compounds are called heterocyclic aromatic compounds. Some examples are as follows:
(a) Benzenoid aromatic compounds
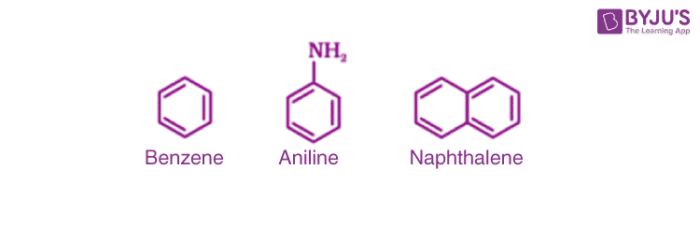
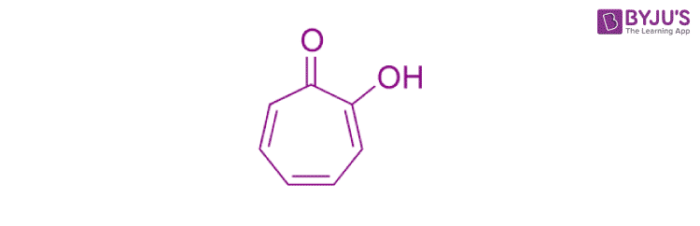
(b) Non-benzenoid aromatic compounds
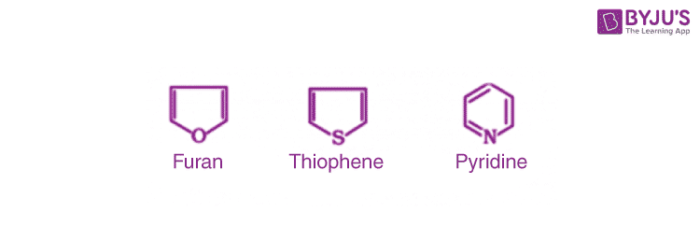
4. Heterocyclic Aromatic Compounds
Organic compounds can also be classified on the basis of functional groups into families or homologous series.
1. Functional group
The functional group can be defined as an atom or a group of atoms that are joined together in a specific manner, which is responsible for the characteristic chemical properties of organic compounds. Examples, in this case, are the hydroxyl group -OH, aldehyde group -CHO and carboxylic acid group -COOH.
2. Homologous series
A group or a series of organic compounds in which each member contains the same characteristic functional group and differs from each other by a fixed unit form a homologous series and therefore its members are known as homologous. The members of the homologous series can be represented by a general formula and the successive members differ from each other in the molecular formula by a CH2 unit. There are a number of homologous series in organic chemistry such as alkanes, alkenes, alkynes, haloalkanes, alkanols, amines, etc.
Detection of Carbon and Hydrogen
Frequently Asked Questions – FAQs
What is meant by organic compounds?
Organic compound is of a large class of chemical compounds in which one or more carbon atoms are covalently paired with other elements atoms, most commonly hydrogen, oxygen, or nitrogen.
What is an example of an organic chemical?
Types include gasoline, plastics, detergents, colourants, food additives, natural gas, and drugs. Soap and detergent are two different examples of organic chemistry, although both are used for washing.
What are the uses of organic compounds?
Organic molecules are used in a variety of industries in human society, including food, pharmaceuticals, fuels, and building, to name but a few. Alkanes include chemical substances such as propane, octane, and methane. These are commonly used as oils for items like gasoline in the car and heating/cooking oil in the home.
Why are organic compounds useful?
Organic compounds are essential because they contain carbon in all living organisms. They are the basic components that move the world in many of the cycles. For example, the carbon cycle which involves exchanging carbon in photosynthesis and cell respiration between plants and animals.
What organic compounds are used in medicine?
Compounds used as medicinal products are most usually organic compounds, sometimes divided into large groups of small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and “biologics” (infliximab, erythropoietin, insulin glargine), the latter more widely used as protein medicinal products.
What are organic and inorganic compounds?
Organic chemistry is the study of the carbon compounding molecules. Inorganic chemistry, by contrast, is the study of all compounds that do NOT contain carbon compounds.
How do you know if a compound is organic?
A material is organic if it contains carbon bound to other atoms by covalence. The other atoms most commonly contain hydrogen, oxygen, and/or nitrogen. A few carbon compounds, including simple oxides (e.g. CO2) and cyanides (e.g. KCN), are arbitrarily omitted.
What are simple organic compounds?
The simplest organic compounds are the hydrocarbons, which only contain hydrogen and carbon. Alkanes only contain single carbon-hydrogen and carbon-carbon bonds, alkenes contain at least one double carbon-carbon bond, and alkynes contain one or more triple carbon-carbon bonds.
Where do organic compounds come from?
An organic compound is a member of a class of chemicals containing carbon atoms bound to one another and other atoms by covalent bonds and found in the cells of living organisms. Hydrogen, oxygen and nitrogen are typical elements which, in addition to carbon, make up organic compounds.
How can you identify an organic compound?
Organic compound, any of a large class of chemical compounds in which one or more carbon atoms are covalently bound to atoms of other elements, most commonly hydrogen, oxygen or nitrogen. The only carbon-containing compounds not known as organic include carbides, carbonates and cyanide.
What are the properties of organic compounds?
Usually, the physical properties of organic compounds of interest provide both quantitative and qualitative characteristics. Quantitative information includes the melting point, the boiling point and the refraction index. Qualitative properties include odour, durability, solubility and colour.
This article deals with the classification of organic compounds. For any further detail on this topic install BYJU’S – the learning app.


Tans for ur teaching, i appreciate so much
So useful for my notes.. Really thank you.. God bless your channel.
It’s very useful for me and other thank you so much
wow I like it its amaizing thnx alot
very useful thanks for helping with my homework!