What is Magnesium Carbonate?
MgCO3 is an inorganic salt with the chemical name Magnesium Carbonate. It is also called Magnesite Hydromagnesite or Barringtonite. Hydrated forms of magnesite such as di, tri, and tetrahydrates are present as minerals. It acts as a fertiliser and as an antacid. It is widely used in the manufacturing of materials that are capable of withstanding extremely high temperatures.
Magnesium carbonate is a basic hydrated magnesium carbonate or a normally hydrated magnesium carbonate. It occurs as light, white, friable masses or as a bulky white powder. It is odourless and is stable in the air. It is practically insoluble in water to which however it imparts a slightly alkaline reaction. It is insoluble in alcohol but is dissolved by dilute acids with effervescence.
Hydromagnesite is a white or yellowish or greyish-white or brown coloured compound which is obtained in crystalline powder or crystalline solid form. It is an important ore for magnesium. It is a carbonate salt, a one-carbon compound, and a magnesium salt.
Table of Contents
- Properties of Magnesium Carbonate- MgCO3
- Magnesium Carbonate Structure- MgCO3
- MgCO3 Uses ( Magnesium Carbonate)
- Important Questions
- Production of Magnesium Carbonate
- Health hazards
- Frequently Asked Questions – FAQs
Properties of Magnesium Carbonate – MgCO3
- Heavy magnesium carbonate is available as a granular powder whereas light magnesium carbonate is available as a very light powder. Both are white, odourless and tasteless.
- They are insoluble in water and alcohol and are soluble in mineral acids with effervescence.
- When heated they are converted to MgO, losing carbon dioxide and water.
| MgCO3 | Magnesium Carbonate |
| Molecular weight of MgCO3 | 84.3139 g/mol (anhydrous) |
| Density of Magnesium Carbonate | 2.958 g/cm3 (anhydrous) |
| Melting point of Magnesium Carbonate | 350 °C |
| Boiling point of Magnesium Carbonate | Decomposes |
Magnesium Carbonate structure – MgCO3
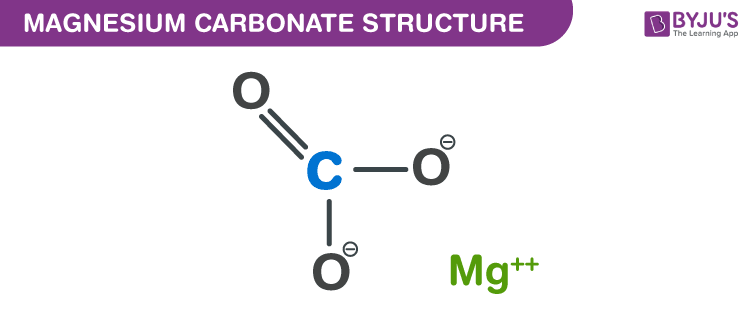
Structure of Magnesium Carbonate
MgCO3 Uses (Magnesium Carbonate)
A list of uses of Magnesium carbonate is given below.
- Magnesium carbonate is used in food as a drying agent.
- Used in making pharmaceutical products.
- Used as a rubber reinforcing agent.
- Used in the manufacturing of cosmetics.
- Used in making mineral water.
- Used as an anti-caking agent in food.
- Used as a filtering agent.
- Used as fire-extinguishing.
- Used in printing inks.
Important Questions
What is magnesium carbonate used for?
Magnesium carbonate is mainly used in processing magnesium oxide by calcining. Magnesite and dolomite minerals are used to make refractory bricks. MgCO3 is also used in fire extinguishing, flooring, cosmetics, dusting powder, fireproofing, and toothpaste.
Is magnesium carbonate toxic?
Magnesium carbonate, a chemical compound with the formula MgCO3, is not toxic to human beings. This inorganic salt of magnesium is a white solid under ambient conditions.
How is magnesium carbonate produced?
Typically, magnesium carbonate is obtained via the mining of the mineral magnesite. In China, 70 percent of the supply worldwide is mined and prepared. In the laboratory, magnesium carbonate can be prepared by reaction of any soluble magnesium salt and sodium bicarbonate.
Production of Magnesium Carbonate
1. It can be prepared in the laboratory by reacting with any soluble sodium bicarbonate and magnesium salt. The reaction is as follows:
MgCl2(aq) + 2NaHCO3(aq) → MgCO3(s) + 2NaCl(aq) + H2O(l) + CO2(g)
2. If sulphate or magnesium chloride is treated with aqueous sodium carbonate, a hydrated complex of magnesium carbonate as well as magnesium hydroxide, a precipitate of magnesium carbonate, is formed. The reaction is as follows:
5MgCl2(aq) + 5Na2CO3(aq) + 5H2O(l) → Mg(OH)2·3MgCO3·3H2O(s) + Mg(HCO3)2(aq) + 10NaCl(aq)
3. Industrially it can be obtained by combining a slurry of magnesium hydroxide and carbon dioxide at moderate temperature and high pressure. On vacuum drying the bicarbonate, it loses carbon dioxide (CO2) and water molecules:
Mg(OH)2 + 2 CO2 → Mg(HCO3)2
Health hazards
- When magnetise is inhaled or comes in contact with the eyes and skin it causes irritation.
- Magnesium carbonate is used as an antacid which neutralises or reduces stomach acid.
- It helps relieve symptoms of excessive stomach acidity in patients with indigestion, heartburn or gastro-oesophageal reflux disorder.
- Magnesium carbonate appears to be well absorbed compared to magnesium oxide and chloride.
- Major side effects include decreased alertness, headache, drowsiness or dizziness, loss of appetite, nausea, vomiting and weakness.
Frequently Asked Questions-FAQs
1. What are the uses of magnesium carbonate?
Ans: Magnesium carbonate is used to fight depression, reduce blood pressure, help prevent migraines, reduce insulin resistance and boost exercise performance,
2. What are the side effects of magnesium carbonate?
Ans: General. Magnesium toxicity symptoms include hypotension, nausea, vomiting, facial flushing, urinary retention, ileus, anxiety, and lethargy, which may lead to muscle weakness, dyspnea, severe hypotension, arrhythmia, and heart arrest.
3. What happens when you heat magnesium carbonate?
Ans: When we heat magnesium carbonate it produces MgO(s) and CO2(g)
4. Does magnesium carbonate help you sleep?
Ans: It helps to regulate the quality of sleep and also magnesium assists you to get to sleep, but it also helps you get to profound and restful sleep.
5. What is magnesium carbonate hydrate?
Ans: Magnesium Carbonate Hydrate is a water-insoluble source of magnesium that can be readily transformed by heating (calcination) to other magnesium compounds. When handled with diluted acids, carbonate compounds also release carbon dioxide.
Learn more about the structure, physical and chemical properties of MgCO3 from the experts at BYJU’S.

Comments