According to texts in the books of inorganic chemistry, bicarbonates are the intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with HCO3– as its chemical formula. Bicarbonates serve an essential biochemical role in the physiological pH buffering based systems.
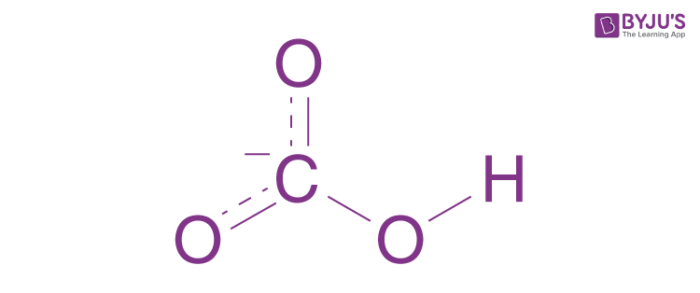
Table of Contents
- Structural relationship to carbonic acid and bicarbonate anion
- Some compounds of Bicarbonates
- Uses of Bicarbonates
- Importance Of Bicarbonates To Human Health
- Frequently Asked Questions- FAQs
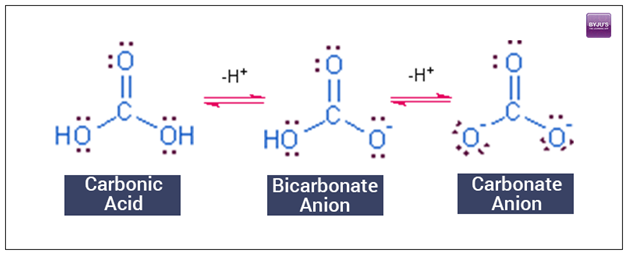
Structural relationship to carbonic acid and bicarbonate anion
The balance of acid-base composition is maintained and controlled by the capability of the respiratory system to eliminate excess carbon dioxide along with the ability of the kidneys to remove wastes in urine and buffering system which is referred to as the buffering system of bicarbonates. The appropriate availability of bicarbonates is the biggest limiting factor to neutralise acidic waste in the human body, which serves as an antioxidant. Bicarbonates can also be delivered via water, in fact, neither water nor the blood is healthy without them.
Neutralisation of acids is based on the existence of buffers. For example, if a weak acid is added to a glass of water that has a zero buffering capacity, the pH drops immediately. But, if the same amount of acid is added to a glass filled with buffered water, the pH remains same as before. The buffer neutralises the acid and leaves the pH of the solution same. The buffers are typically bicarbonates in water and within the human body.
The bicarbonates buffering systems are composed of weak acid i.e, carbonic acid(H2CO3) and a weak base i.e, bicarbonate(HCO3). When combined, they function to keep the pH of the blood and other internal fluids in a particular range. The carbonic acid in the solution dissociates into hydrogen ions and bicarbonate ions. When the acid is added to a bicarbonate system, the bicarbonate ions reconnect with H+ ions and form carbonic acid again. This reduces the concentration of H+ ions and brings the pH back to its original value. The buffers neutralize acids by eliminating hydrogen ions.
Some compounds of Bicarbonates
1. Sodium Bicarbonate
- The molecular formula of sodium bicarbonate is NaHCO3.
- It’s also named as baking soda as on heating it produces bubbles of carbon dioxide which makes cakes or pastries light and fluffy by making holes in them.
- Preparation: Sodium bicarbonate is prepared by saturating a solution of NaCO3 with carbon dioxide; in this process NaHCO3 separates as white crystal.
Na2CO3 + CO2 + H2O → 2NaHCO3
- Physical state and Solubility: It is a white crystalline solid, sparingly soluble in water. The solution is alkaline in nature due to hydrolysis. This solution gives yellow colour with methyl orange but no colour with phenolphthalein.
- Uses: It is used as a mild antiseptic for skin infections, antacids to neutralise the acidity in the stomach, fire extinguishers and for making baking powder.
Also Read : Sodium Bicarbonate, Baking Soda
2. Potassium Bicarbonate
- The molecular formula of potassium bicarbonate is KHCO3.
- It is white crystalline solid soluble in water.
- Preparation: Potassium bicarbonate is prepared by treating an aqueous solution of potassium carbonate with carbon dioxide.
K2CO3 + CO2 + H2O → 2 KHCO3
- Uses: This compound is a source of carbon dioxide. It can substitute for baking soda (sodium bicarbonate) for those with a low-sodium diet, it is widely used as a pH regulated reagent or buffering agent in medications and an additive in winemaking.
Also Read : Potassium bicarbonate
2. Ammonium Bicarbonate
- The molecular formula of ammonium bicarbonate is NH4HCO3
- Ammonium hydrogen carbonate is a crystalline solid white in colour which smells like ammonia. It is soluble in water but insoluble in ethanol.
- Preparation: Ammonium hydrogen carbonate is obtained by combining carbon dioxide (CO2) and ammonia (NH3). This compound is thermally unstable and the reaction solution is maintained cold to allow the precipitation as a white solid
CO2 + NH3 + H2O → (NH4)HCO3
- Uses: Ammonium bicarbonate is used in the food industry as a food additive, in fire extinguishers, in the manufacturing of dyes, as a fertiliser and produces ammonium salt
Also Read : Ammonium Bicarbonate
Uses of Bicarbonates
- While baking bicarbonates like sodium bicarbonate find a role as a leavening agent.
- They are used to increase the internal pH of the stomach to counteract the activity of highly acidic digestive juices.
- Bicarbonate is a vital component of the pH buffering system of the human body.
- Ammonium bicarbonate is used in digestive biscuit manufacture.
Importance Of Bicarbonates To Human Health
The Bicarbonates provide blood with the ability to resist changes in pH. Any minute variations in the pH of blood can disrupt critical metabolic functions. Bicarbonates also contribute in resisting the impacts of acid rain in natural water and other acidic influences. The dramatic swing in pH of streams and rivers can be equally destructive. The ocean is also buffered with bicarbonates and capable of neutralising the enormous amount of acidic waste.
There is much more bicarbonate compared to carbonic acid (approximate ratio 20:1) under the ideal condition in the human body. This is suitable for organisms that release metabolic waste. The higher concentration of bicarbonate at equilibrium is capable of neutralising the significant amount of acidity. However, bicarbonates become the limiting factor under stress. Pollution, diet, exercise, and age draw bicarbonates and restrict the body’s capacity to neutralise and eradicate acids.
Frequently Asked Questions- FAQs
1. What is bicarbonate used for?
Bicarbonate is used to increase the internal pH of the stomach to counteract the activity of highly acidic digestive juices. It is used as a leavening agent in baking. Ammonium bicarbonate is used in digestive biscuit manufacture.
2. What happens when you mix baking soda and lemon juice?
When baking soda is mixed with lemon juice, bubbles are formed. These bubbles are formed due to the evolution of carbon dioxide gas.
3. What is the difference between carbonate and bicarbonate?
Even though sodium carbonate and sodium bicarbonate sound similar in their names, these two substances are not the same and have many features and uses that are different. Sodium carbonate is often referred to as soda ash or washing soda. Sodium bicarbonate is popularly called baking soda.
4. Why do athletes take sodium bicarbonate?
During high-intensity workouts, our body releases lactic acid. This acidity is directly related to pH levels in our bodies causing our muscles to burn and feel fatigued. Sodium bicarbonate clears the acid out of muscle cells, helping restore an optimal pH. So that Sodium bicarbonate (NaHCO3) supplementation is given to athletes to improve their performance.


Comments