What is Ammonium bicarbonate?
NH4HCO3 is an inorganic compound with chemical name Ammonium bicarbonate. It is also called Ammonium hydrogen carbonate or Monoammonium carbonate or Ammonium hydrogen carbonate.
It is the bicarbonate salt (HCO−3) of the ammonium ion (NH⁺₄) which degrades readily to CO2 (carbon dioxide), ammonia, and water.
Table of Contents
- Ammonium bicarbonate Structure
- Production of Ammonium bicarbonate
- Properties of Ammonium bicarbonate
- Uses of Ammonium bicarbonate
- Health Hazards of Ammonium bicarbonate
- Frequently Asked Questionss – FAQs
Ammonium bicarbonate structure – NH4HCO3
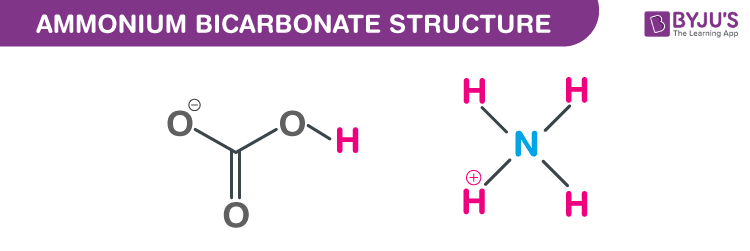
Ammonium hydrogen carbonate is a colourless crystalline solid which smells like ammonia. It is soluble in water but insoluble in ethanol, acetone, alcohol, and benzene. It is hazardous to the environment and immediate measures should be taken to stop the spread. It is widely used in food processing.
Properties of Ammonium bicarbonate – NH4HCO3
| NH4HCO3 | Ammonium bicarbonate |
| Molecular weight of NH4HCO3 | 79.056 g/mol |
| Density of Ammonium bicarbonate | 1.586 g/cm3 |
| Flash point of Ammonium bicarbonate | Non-flammable |
| Melting Point of Ammonium bicarbonate | 41.9 °C |
NH4HCO3 Uses (Ammonium bicarbonate)
- Ammonium bicarbonate is used in the food industry as a food additive.
- Used in fire extinguishers.
- Used in the manufacturing of dyes.
- Used as a fertilizer.
- Used to produce ammonium salt.
- Used in the manufacturing of pharmaceutical products.
- Used in the making of paints.
- Used in the manufacturing of ceramics.
- Used in leather tanning.
- Used in cooling baths.
Production of Ammonium bicarbonate
Ammonium hydrogen carbonate is obtained by combining carbon dioxide (CO2) and ammonia (NH3):
CO2 + NH3 + H2O → (NH4)HCO3
This compound is thermally unstable and the reaction solution is maintained cold to allow the precipitation as a white solid. In the year 1997, almost 1,00,000 tons were produced by the above process.
Health hazards
Inhaling Monoammonium carbonate causes respiratory irritation. Swallowing is harmful and contact with skin or eyes causes severe irritation. When heated, irritating, toxic ammonia gas will be liberated from the compound. It decomposes with explosion and ammonia gas is produced.
Frequently Asked Questions – FAQs
What are the uses of ammonium bicarbonate?
In the food industry, ammonium bicarbonate is used as a leavening agent for flat baked goods, including cookies and crackers. Until modern-day baking powder was made available, this compound was widely used in homes for baking purposes.
Is ammonium bicarbonate toxic?
Ammonium Carbonate is a crystalline, non-toxic, white-coloured salt with the molecular formula (NH4)2CO3. It is also known as baker’s ammonia and also as hartshorn. Ammonium carbonate is water-soluble, and the compound decomposes in hot water.
How is ammonium bicarbonate produced?
Ammonium bicarbonate can be produced by reacting ammonia with carbon dioxide in the presence of water. Since this compound is thermally unstable, the reaction conditions must be kept relatively cold.
What are the benefits of ammonium bicarbonate?
Ammonium Bicarbonate is an inorganic substance that is the bicarbonate salt of the ammonium ion in chemical terms. This component is utilised as a leavening and stabilising agent, as well as an acidity regulator, due to its physicochemical qualities.
What is the property of NH4HCO3?
Ammonium bicarbonate (NH4HCO3) is an inorganic compound with the chemical formula NH4HCO3. Ammonium hydrogen carbonate is a white crystalline substance with an ammonia-like odour. Water dissolves it, while ethanol, acetone, alcohol, and benzene do not.
Learn more about the Structure, physical and chemical properties of NH4HCO3 from the experts at BYJU’S.

thank u . it is very helpful .