What is Barium Sulphate (BaSO4)?
BaSO4 is an inorganic compound with the chemical name Barium Sulphate.
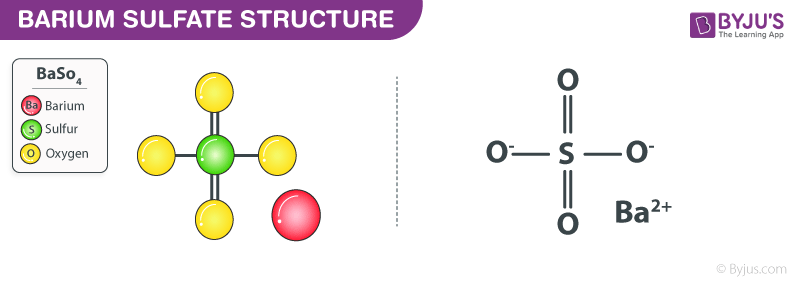
Barium Sulphate is composed of a barium cation and the sulphate anion. The sulphur is attached to four oxygen atoms. BaSO4 is a sulphate salt of barium and is found as the mineral barite. It is a crystalline solid white which is insoluble in water and alcohol but soluble in concentrated acids. It is odourless.
Barium Sulphate is an alkaline, divalent metal. It is non-toxic and safe for medical use. It is widely used in the production of oil and natural gas to get high-density drilling fluids by keeping the boreholes free of rock.
Properties of Barium Sulphate – BaSO4
| BaSO4 | Barium Sulphate |
| Molecular Weight/ Molar Mass | 233.38 g/mol |
| Density | 4.5 g/cm³ |
| Boiling Point | 1,600 °C |
| Melting Point | 1,580 °C |
Barium Sulphate Structure (BaSO4 Structure)
Barium Sulphate (BaSO4) Uses
- It is used in the casting of copper anode plates as a coating material.
- It is used as a radiopaque agent.
- It is used as a component in oil well drilling fluids.
- It is used to increase the density of the polymer by acting as a filler for plastics.
- Used to test the pH of the soil.
- Used as a filler or to modify the consistency in oil paints.
- It is used in imaging the GI tract during barium meals.
- Used to diagnose certain disorders of the intestines, stomach, or esophagus.
- It is used in the manufacturing of alloys.
Frequently Asked Questions
What are the side effects of barium sulphate?
Common side effects can include minor cramps in the stomach; nausea, vomiting; or. Stools loose, or moderate constipation. Extreme cramping, diarrhoea or constipation; ringing in the ears; sweating, anxiety, rapid heart rate; or light skin, grey eyes, fatigue.
Is barium sulphide soluble in water?
For uses such as water treatment, most metal sulphate compounds are readily soluble in water, unlike the fluorides and oxides that appear to be insoluble.
Is barium sulphate soluble in hexane?
Sodium sulphate is a white powder that is highly soluble in water but not soluble in hexane (a hydrocarbon liquid). Dissolved wat, if added to the barium nitrate. Sodium sulphate is a white powder that is highly soluble in water but not soluble in hexane (a hydrocarbon liquid).
How do you make barium sulphate?
The barium sulphate is prepared by sodium sulphate reacting to barium chloride. The precipitate forms the barium sulphate.
Is barium sulphate an acid or base?
BaSO4 is known as barium sulphate. This is salt. Salt can be formed from the acids and base reaction. If we monitor the BaSO4 development reaction, we can see that Ba^2 + comes from the base and that SO4^2- comes from the acid.
Other related links:
| Barium | Properties Of Alcohols |


Comments