What is Copper Sulfate?
Copper sulfate is a term that can refer to either of the following chemical compounds – cuprous sulfate (Cu2SO4), or cupric sulfate (CuSO4). However, the latter is the preferred compound described by the term ‘copper sulfate’. The systematic name for CuSO4 is copper(II) sulfate, but it is also referred to as blue vitriol, Roman vitriol, the vitriol of copper, and bluestone.
The most common form of copper sulfate is its pentahydrate, given by the chemical formula CuSO4.5H2O. This form is characterized by its bright blue colour. However, it can be noted that the anhydrous form of this salt is a powder that is white.
The CuSO4 molecule consists of an ionic bond between the copper cation (Cu2+) and the sulfate anion (SO42-). An illustration describing the structure of a copper sulfate molecule is provided below.
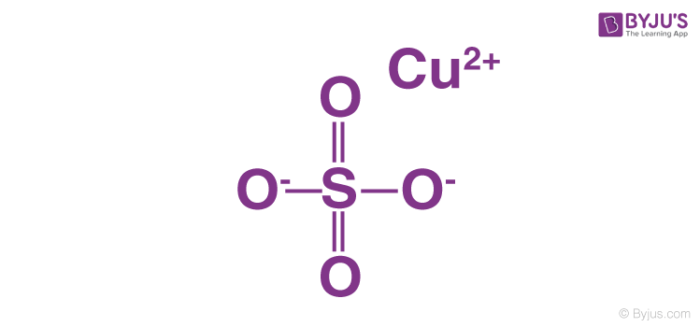
Copper sulfate can be prepared by treating metallic copper with heated and concentrated sulphuric acid, or by treating the oxides of copper with dilute sulphuric acid. It can be noted that the oxidation state exhibited by the copper atom in a CuSO4 molecule is +2.
Properties of CuSO4
The physical and chemical properties of copper sulfate are discussed in this subsection. It can be noted that the properties of anhydrous CuSO4 and CuSO4.5H2O vary considerably, and have been highlighted separately.
Physical Properties
- The molar mass of the anhydrous and the pentahydrate forms of copper sulfate are 159.609 grams/mole and 249.685 grams per mole respectively.
- Anhydrous CuSO4 has a grey-white, powdery appearance whereas the pentahydrate has a bright blue colour.
- The densities of the anhydrous and pentahydrate forms are 3.6 grams per cubic centimetre and 2.286 g.cm-3
- Both hydrated and anhydrous copper sulfates tend to decompose on heating and hence do not have exact boiling points.
- Anhydrous CuSO4 has an orthorhombic crystal structure whereas CuSO4.5H2O crystals have triclinic structures.
Chemical Properties
- The copper ions present in copper sulfate react with the chloride ions belonging to concentrated hydrochloric acid, leading to the formation of tetrachlorocuprate(II).
- The chemical equation for this reaction is given by Cu2+ + 4Cl– → CuCl42-
- When heated to 650oC, CuSO4 undergoes a decomposition reaction to yield cupric oxide (CuO) and SO3 (sulfur trioxide).
- Copper sulfate is highly soluble in water, with solubility values of 1.055 molal and 1.502 molal ate 10oC and 30oC respectively.
A typical example of a single displacement reaction where one metal displaces another is the reaction between iron and copper sulfate, given by the reaction Fe + CuSO4 → FeSO4 + Cu
Uses of Copper Sulfate
Basic chemistry sets that are used as educational tools generally include copper sulfate. The chemical compound CuSO4 has a wide range of applications. Some of these uses are listed below.
- The pentahydrate of this compound, CuSO4.5H2O is used as a fungicide due to its ability to kill several fungi.
- Copper sulfate is used in Benedict’s solution and in Fehling’s solution, which is used in testing for reducing sugars.
- It is also used to test blood samples for diseases like anaemia.
- CuSO4 is mixed with KMnO4 (potassium permanganate) to form an oxidant which can be used in the conversion of 1o
- It is also used as a dye fixative in the process of vegetable dyeing.
- Solutions of copper sulfate in water can be used as a resistive element liquid resistors.
- It can also be used as a decorative since it can add colour to cement, ceramics, and other metals as well.
- Copper sulfate is also added to bookbinding glues in order to protect the printed paper from insects.
Frequently Asked Questions
What is copper sulphate used for?
The compound’s pentahydrate, CuSO4. 5H2O is used as a fungicide because it can destroy many fungi. Copper sulfate is used in Fehling’s and Benedict’s solutions. Blood samples can be tested for conditions such as anaemia with the help of this compound.
Why is anhydrous copper sulphate white and the pentahydrate blue?
In hydrated CuSO4, the water molecules surrounding the Central Metal (Cu) act as ligands resulting in d-d transition and therefore emitting blue colour in the visible region due to which hydrated CuSO4 appears blue. Since anhydrousCuSO4 does not hold any water of crystallization, It retains its white colour.
Is copper sulphate solid or aqueous?
Copper(II) sulfate is a hydrated, blue solid – it is attached to water molecules. This becomes whitish when anhydrous – when it is not molecularly bound to water. When it is hydrated, there are usually five molecules of water attached to one cooper sulphate molecule. Heating up the CuSO4 will dehydrate it.
From the uses described above, it can be understood that copper sulfate is an extremely important chemical compound, despite its toxicity to human beings. To learn more about CuSO4 and other important chemical compounds, such as K2Cr2O7, register with BYJU’S and download the mobile application on your smartphone.


Comments