What is Potassium Permanganate?
Potassium Permanganate (KMnO4) is an inorganic chemical compound. It is also known as Condy’s crystals or permanganate of potash.
A German-Dutch chemist Johann Rudolf Glauber was the first to discover the production of KMnO4 in the year 1659. This compound is water-soluble and consists of two ions: Permanganate ion and potassium ion. It is a dark purple odourless solid in its physical state.
When potassium permanganate crystals are dissolved in water the solution formed is purple. It is considered as a strong oxidizing agent and does not produce toxic by products. It is usually prepared from other minerals such as manganese oxide.
Table of Contents
- Structure of Potassium Permanganate
- Preparation of Potassium Permanganate
- Physical Properties of Potassium Permanganate
- Chemical Properties of Potassium Permanganate
- Reactions of Potassium Permanganate
- Uses of Potassium Permanganate
- Effects on Health
- Solved Example
- Frequently Asked Questions – FAQs
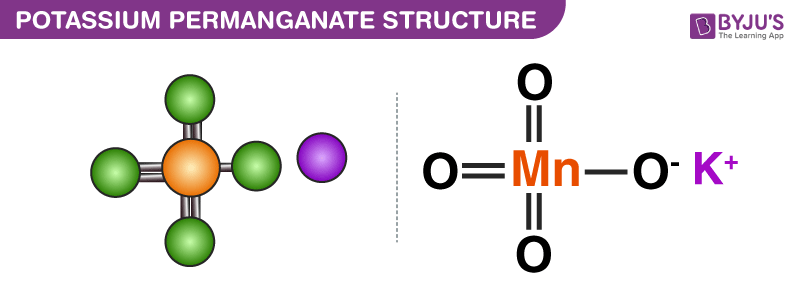
Structure of Potassium Permanganate (KMnO4)
The structure of potassium permanganate molecules is illustrated below. Note that this compound features an ionic bond between the potassium cation and the permanganate anion.
Preparation Of Potassium Permanganate – KMnO4
Potassium permanganate is commercially prepared by mixing solution of KOH and powdered manganese oxide, with oxidizing agents like potassium chlorate. The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency.
6KOH + 3MnO2 + 6KClO3 → 3K2MnO4 + 6KCl + 3H2O
The potassium manganate (green) so formed is boiled with a large quantity of water and current of chlorine, CO2 and ozonized air is passed into the liquid until it is converted into permanganate. The MnO2 formed is removed continuously in order to prevent its breaking down the permanganate.
6K2MnO4 + 3Cl2 → 6KMnO4 (Potassium Permanganate) + 6KCl
The solution of KMnO4 is drawn off from any precipitate of MnO2 concentrated and crystallized. The crystals are centrifuged and dried.
Physical Properties Of Potassium Permanganate – KMnO4
- It is an odourless, purple to magenta crystalline solid.
- It is soluble in water, acetone, acetic acid, methanol, and pyridine.
- It gets dissolved in ethanol and organic solvents.
- Potassium permanganate occurs in the form of monoclinic prisms, almost opaque with a blue metallic lustre.
- It is odourless. An aqueous solution has a sweetish astringent taste. It is water-soluble and more soluble in boiling water.
Chemical Properties Of Potassium Permanganate
- Potassium permanganate is a very strong oxidizing agent and can, therefore, be used as an oxidant in a wide spectrum of chemical reactions.
- The oxidizing power of potassium permanganate can be seen while performing a redox reaction with it, in which the dark purple solution turns colourless and then into a brown solution.
- The above reaction can be performed in an acidic or a basic medium.
| KMnO4 | Potassium permanganate |
| Compound Name | Potassium manganate(VII) |
| Molecular Weight/ Molar Mass of Potassium permanganate | 158.034 g/mol |
| Density of Potassium permanganate | 2.703 g/cm³ |
| Storage temperature of Potassium permanganate | Room temperature |
| Boiling Point of Potassium permanganate | 100oC |
| Melting Point of Potassium permanganate | 240°C |
| Oxidation State | +7 |
Reactions Of Potassium Permanganate (KMnO4)
1. Thermal decomposition:
When solid potassium permanganate is heated it undergoes decomposition. The reaction is as follows:
2KMnO4 → K2MnO4 + MnO2(s) + O2
2. Reaction with acid:
When permanganate reacts with concentrated hydrochloric acid it produces chlorine. In a neutral solution, permanganate is reduced by three electrons to produce manganese dioxide, where the oxidation state of manganese is +4. Potassium permanganate reduces spontaneously in an alkaline solution and turns into green K2MnO4.
3. Effect of Alkalies
On heating with alkalies, potassium permanganate changes into manganate and oxygen gas is evolved.
4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2
4. Oxidizing properties
KMnO4 acts as a very powerful oxidizing agent in acidic, neutral and alkaline media. The equations representing oxidation in these media are
In acidic medium
2KMnO4 + H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O] MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
In neutral or alkaline medium
2KMnO4 + H2O → 2KOH + 2MnO2 + 3[O]
MnO4– + 2H2O + 3e– → MnO2 + 4OH–
Uses Of Potassium Permanganate (KMnO4)
There are wide applications of KMnO4. Some important uses of potassium permanganate have been discussed below:
- Potassium permanganate is used in qualitative analysis to determine the permanganate value
- KMnO4 is also used as a regeneration chemical in well water treatment for the removal of hydrogen sulphide and iron
- This compound is also used as a disinfectant to cure certain skin conditions like foot fungal infections, dermatitis
- Another important application of potassium permanganate is in the treatment of bacterial infections
- KMnO4 is also known to be used in tanning leathers, printing fabrics
- This compound can even be used as a bleaching agent, as a pesticide, and as an antiseptic
- One of the most important industrial applications of potassium permanganate is as an oxidizing agent in the chemical synthesis of many important compounds.
Effects on Health
- In a concentrated form, potassium permanganate is an irritant to human eyes and skin. It can react with many reducing agents or organic material but it is inflammable.
- The antibacterial action of KMnO4 is dependent on the oxidation of proteins of bacteria or tissues by this compound. It leaves a stain on skin or tissues. Since it acts by destructive oxidation process on all organic matter, its use is restricted for external purposes only.
- Potassium permanganate acts as an antidote in barbiturates, chloral hydrate, and alkaloidal poisoning. A solution of 1:5000 of permanganate when used as a gastric wash, oxidizes poison and prevents their absorption.
- This compound is usually stored in tightly closed containers. Potassium permanganate should be handled with care since an explosion may occur when it comes in contact with readily oxidizable substances.
Solved Example
Question:
Give the ionic equation for the oxidation of iodides with KMnO4 in acidic medium and in alkaline medium.
Solution:
In acidic medium:
[MnO4– + 8H+ + 5e– → Mn2+ + 4H2O ] x 22I– →
I2 + 2e– ] x 5
———————————————————————–
10I– + 2MnO4– + 16H+ → 2Mn2+ + 5I2 + 8H2O
———————————————————————–
In alkaline medium:
MnO4– + 2H2O + 3e– → MnO2 + 4OH– ] x 2
I– + 6OH– → IO3– + 3H2O + 6e–
————————————————————————
2MnO4– + I– + H2O → IO3– + 2MnO2 + 2OH–
Frequently Asked Questions – FAQs
Write the formula for potassium permanganate
The chemical formula of potassium permanganate is KMnO4.
Permanganate a good oxidizing agent. Why?
As the oxidation states of atoms increase the elements become more electronegative. Therefore, permanganate a good oxidizing agent.
List two uses of potassium permanganate.
Two uses of potassium permanganate are-
It is used to treat various skin diseases such as fungal infection in the foot
It is used as an oxidizing agent in industries.
What is the colour of potassium permanganate?
Potassium permanganate’s physical state is an odourless solid, and they look like crystals of dark purple or bronze colour. When these crystals are dissolved in water, the solution becomes purple.
Why KMnO4 is a self indicator?
The solution under examination loses its pink colour once all the permanganate ions are used up in the reaction. It suggests the end of the reaction and therefore a self-indicator is called potassium permanganate, as it serves as an indicator apart from being one of the reactants.
Learn more about the chemical behaviour and importance of KMnO4 with the expert faculties at BYJU’S.
Read more:


Comments