What is Calcium acetate?
C4H6CaO4 is a calcium salt of acetic acid with the chemical name Calcium acetate.
Calcium acetate is also called Acetate of lime or Calcium ethanoate or Calcium diacetate. It is widely used as a hydrate in treating hyperphosphataemia and functions as a chelator.
It is hygroscopic in its anhydrous form and monohydrate in the common form. A mineral such as calcium is required for many cellular functions such as nerve impulse transmission, cardiac function, cell membrane permeability, muscle contraction, and bone formation. Calcium acetate is taken orally to treat or prevent calcium deficiency as well as to treat hyperphosphatemia as it has excellent phosphate-binding properties.
Table of Contents
- Properties of Calcium acetate
- Calcium acetate Structure
- Calcium acetate Uses
- Calcium acetate Production
- Calcium acetate Health Hazards
- Frequently Asked Questions – FAQs
Properties of Calcium acetate – C4H6CaO4
| C4H6CaO4 | Calcium acetate |
| Molecular weight of C4H6CaO4 | 158.166 g/mol |
| Density of Calcium acetate | 1.509 g/cm3 |
| Melting point of Calcium acetate | 160 °C |
| Refractive index | 1.55 |
Calcium acetate Structure – C4H6CaO4
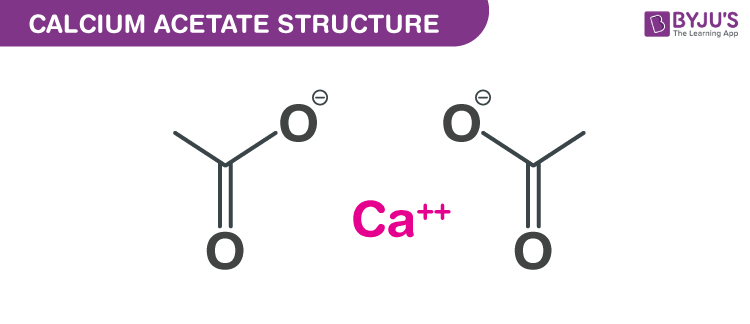
Calcium acetate Structure – C4H6CaO4
Calcium acetate Uses
- Calcium acetate is used as a food additive.
- Used as a corrosion inhibitor.
- Used is the manufacturing of lubricants.
- Used as a stabilizer in resins.
- Used in bakery goods as an anti mold agent.
- Used in gelatin as a thickener.
- Used as an esterification catalyst.
- Used in the making of agricultural products.
- Used as a filler and stabilizing agent.
Calcium acetate Production
Calcium ethanoate can be obtained by soaking hydrated lime or calcium carbonate in vinegar. The reaction is as follows:
CaCO3(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + H2O(l) + CO2(g)
Ca(OH)2(s) + 2CH3COOH(aq) → Ca(CH3COO)2(aq) + 2H2O(l)
Calcium acetate Health Hazards
Acetate of lime is verified and considered to be of low concern based on the experiments conducted and data gathered. It is combustible under fixed conditions.
Frequently Asked Questions – FAQs
What is the use of calcium acetate?
Calcium acetate is used in patients with end-stage kidney failure who are on dialysis to treat hyperphosphatemia with too much phosphate in the blood. Calcium acetate works by binding in the food you consume to the phosphate, and it is extracted from the body without being absorbed.
What are the side effects of taking calcium acetate?
Calcium acetate side effects high calcium levels in your blood — nausea, diarrhoea, constipation, increased appetite or urination, muscle fatigue, bone pain, discomfort, energy loss or tired feeling.
What is the difference between calcium carbonate and calcium acetate?
Two commonly used phosphate-binding agents are calcium carbonate and calcium acetate, and while both have similar efficacy, calcium acetate contains less elemental calcium
Is calcium acetate toxic?
Acute toxicity data for Calcium Acetate indicate that this is level IV toxicity for all exposure paths. Calcium acetate has no subchronic or developmental toxicity and is neither mutagenic nor genotoxic.
Is calcium acetate soluble in water?
Calcium acetate is a water-soluble compound used to neutralize and alkalize paper in aqueous and non-aqueous ways. Nevertheless, calcium acetate is converted to calcium carbonate with a release of acetic acid during the neutralization process.
Learn more about the Structure, physical and chemical properties of C4H6CaO4 from the experts at BYJU’S.


Comments