What is Hydroxide?
OH− is a diatomic anion with the chemical name Hydroxide.
Hydroxide is also called Hydroxyl or Hydroxyl radical or hydroxide ion. It consists of hydrogen and an oxygen atom which are held together by a covalent bond. The hydrogen carries a negative electric charge. It is widely used as a food preservative, for alkalization of urine to prevent kidney stones, as an anticoagulant for stored blood, and buffer. It acts as a ligand, a catalyst, a base, and a nucleophile.
Hydroxide Properties
The ion forms salts where some of them dissociate in an aqueous solution to liberate solvated hydroxide ions. It is a minor constituent of water. When a strongly electropositive center and hydroxide are attached to each other, hydroxide may ionize itself to liberate a hydrogen cation, and makes the parent compound an acid.
HO• which is the electrically neutral compound is the hydroxyl radical. –OH which is the covalently-bound group is the hydroxy group. The hydroxy group and hydroxide ions are nucleophiles and function as catalysts in organic chemistry.
| Hydroxide | OH− |
| Molecular Weight of Hydroxide | 17.007 g/mol |
| Monoisotopic mass of Hydroxide | 17.003 g/mol |
| Conjugate base | Oxide anion |
| Conjugate acid | water |
Structure of Hydroxide (OH−)
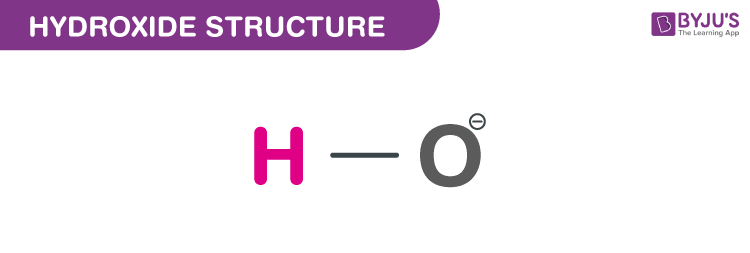
Structure of Hydroxide
Uses of Hydroxide (OH−)
- Hydroxide is used to produce fuel cells.
- Used to produce disinfectants.
- Used as food preservatives to prevent bacteria and mould from growing in food.
- Used in the paper recycling process.
- Used in the extraction of alumina.
All inorganic substances with the word hydroxide in their name do not mean ionic compounds of the hydroxide ion. They are covalent compounds which contain the hydroxy groups.
Frequently Asked Questions
Is hydroxide an acid?
If one of those ions is H+, the solution is acidic. The strong acid hydrogen chloride (HCl) is one example. If one of the ions is OH-, then the solution is base. Sodium hydroxide (NaOH) is one such example of a solid base.
What is the pH of hydroxide?
When a solution’s pH is less than 7, the solution is considered acidic; if the pH is roughly 7, the solution is neutral; if the pH is greater than 7, the solution is considered base. For an acidic solution, then, the hydrogen ion concentration is higher than the hydroxide ion concentration.
Can hydroxide exist on its own?
Hydroxide occurs alone when it is in water since acids and bases can neutralize each other in solution, and it is very important in this situation to distinguish between HO and H2O in reactions.
Is hydroxide harmful?
The concrete is corrosive, and its solutions are. Sodium hydroxide is odourless; thus, the odour does not react to dangerous concentrations. Sodium hydroxide does not produce systemic toxicity but it is very corrosive and can cause extreme burns in all tissues with which it comes into contact.
Why is hydroxide negatively charged?
In this compound, two electrons share oxygen bonds with hydrogen. Hydroxide bears a negative charge since an electron has been absorbed. Oxygen, depicted as O, is bound to a hydrogen, depicted as H, so we can see that the negative sign is the most negative component of the product.
Learn more about the different applications, structure, and properties of Hydroxide (OH−) from the expert faculties at BYJU’S – India’s largest education company.

Comments