What is Ascorbic Acid?
Ascorbic Acid belongs to the monosaccharide family and has a chemical formula C6H8O6. Vitamin C (ascorbic acid) is a key vitamin for animals and plants.
It is a vitamin C and should be obtained in the diet as it cannot be produced by humans. It is also known as Vitamin C or L- ascorbic acid. It is present in citrus fruits, strawberries, broccoli, raw bell pepper, kiwifruit, brussels sprouts etc. It is soluble in water. This organic compound is used as a reducing agent and considered an oxidant.
Ascorbic Acid is also found in leafy vegetables, potatoes, and tomatoes. It is widely used in treating and preventing the common cold. It is applied to the skin to protect it from sun and pollution. People suffering from depression and Alzheimer’s also take vitamin C.
Properties of Ascorbic Acid – C6H8O6
| C6H8O6 | Ascorbic Acid |
| Molecular Weight/ Molar Mass | 176.12 g/mol |
| Density | 1.694 g/cm3 |
| Boiling Point | 553 °C |
| Melting Point | 190 °C |
Ascorbic Acid Structure – C6H8O6
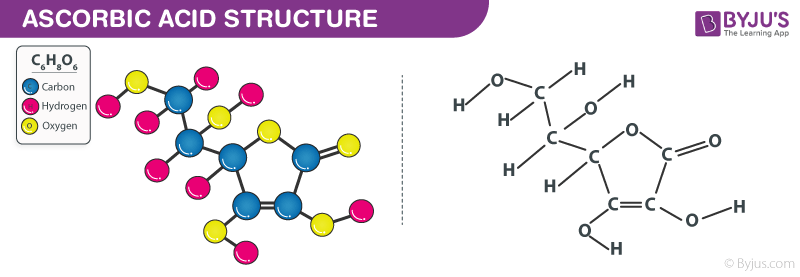
Ascorbic Acid Structure
Uses of Ascorbic Acid
- It is used in the treatment of scurvy
- It is used in the formation of collagen fibres in connective tissue, fibrous tissue, bones, and teeth
- It fights against bacterial infections
- It functions in detoxifying reactions
- It is used in preventing the transfer of HIV from mothers to babies
- It is used in the treatment of acne and gum infection
- It is used to prevent gallbladder disease
- It is used in the treatment of stomach ulcers caused by Helicobacter pylori
FAQs
1. What is ascorbic acid made from?
Some synthetic vitamin C (or ascorbic acid) is produced with fermented corn syrup imported from China, which may or may not has been genetically engineered. To remove the ascorbic acid, the corn syrup is then treated with solvents such as acetone, sulfuric acid or sodium hydroxide.
2. What is ascorbic acid used for?
For bones and connective tissues, muscles, and blood vessels, vitamin C is important. Vitamin C also helps the body absorb iron which is required for the development of red blood cells. Ascorbic acid is used to treat the vitamin C deficiency and avoid it. Ascorbic acid can also be used for purposes not specified in this Guide to Medication.
3. Is ascorbic acid the same as vitamin C?
In fact this means that individual synergistic, including ascorbic acid, can not act as a vitamin in a chemically isolated form. Ascorbic acid isn’t a problem living there. It is a clone of any component of a living complex called vitamin C. Ascorbic acid is a crystalline, fractionated vitamin C isolate.
4. What foods are high in ascorbic acid?
Vitamin C-rich foods include broccoli, cantaloupe, cauliflower, kale, kiwi, orange juice, papaya, red, green and yellow pepper, sweet potatoes, strawberries, and tomatoes.
5. Does ascorbic acid increase stomach acid?
Ascorbic acid has a very high acidity and, when consumed on an empty stomach, can cause these gastrointestinal side effects. Due to the low pH of ascorbic acid, calcium ascorbate (neutralized vitamin C) was developed to reduce the epigastric adverse effect.
Also Read:
| Benzoic Acid | Acetic Acid |
| Sodium Hydroxide | Sulfuric Acid |
Register to BYJU’S to learn more about the properties, applications, and structure of C6H8O6 from the expert teachers.


Comments