What is Acetic Acid?
Acetic acid is an organic compound with the formula CH3COOH. It is a carboxylic acid consisting of a methyl group that is attached to a carboxyl functional group. The systematic IUPAC name of acetic acid is ethanoic acid and its chemical formula can also be written as C2H4O2. Vinegar is a solution of acetic acid in water and contains between 5% to 20% ethanoic acid by volume. The pungent smell and the sour taste is characteristic of the acetic acid present in it.
An undiluted solution of acetic acid is commonly referred to as glacial acetic acid. It forms crystals which appear like ice at temperatures below 16.6oC. It has a wide range of applications as a polar, protic solvent. In the field of analytical chemistry, glacial acetic acid is widely used in order to estimate substances that are weakly alkaline.

Table of Content
Structure of Acetic acid-CH3COOH
- It can be observed in the solid-state of acetic acid that there is a chain of molecules wherein individual molecules are connected to each other via hydrogen bonds.
- Dimers of ethanoic acid in its vapour phase can be found at temperatures approximating to 120o
- Even in the liquid phase of ethanoic acid, its dimers can be found when it is present in a dilute solution. These dimers are adversely affected by solvents that promote hydrogen bonding.
- The structure of acetic acid is given by CH3(C=O)OH, or CH3CO2H
The structure of acetic acid is illustrated below.
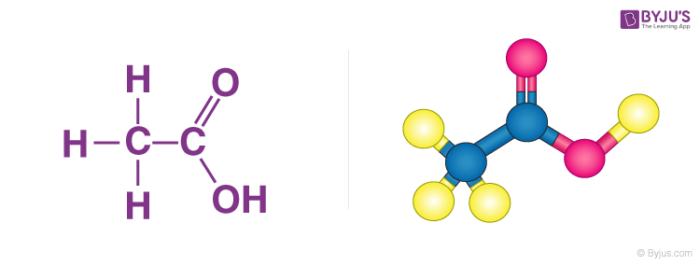
Structurally, ethanoic acid is the second simplest carboxylic acid (the simplest being formic acid, HCOOH), and is essentially a methyl group with a carboxyl functional group attached to it.
Properties of Acetic acid-CH3COOH
| CH3COOH | Acetic Acid |
| Molecular weight/molar mass of CH3COOH | 60.052 g/mol |
| Density of Acetamide | 1.05 g/cm³ |
| Boiling Point of Acetamide | 118 °C |
| Melting Point of Acetamide | 16.6 °C |
Preparation of Acetic acid-CH3COOH
Acetic acid is produced industrially via the carbonylation of methanol. The chemical equations for the three steps involved in this process are provided below.
- CH3OH (methanol) + HI (hydrogen iodide) → CH3I (methyl iodide intermediate) + H2O
- CH3I + CO (carbon monoxide) → CH3COI (acetyl iodide)
- CH3COI + H2O → CH3COOH (acetic acid) + HI
Here, a methyl iodide intermediate is generated from the reaction between methanol and hydrogen iodide. This intermediate is then reacted with carbon monoxide and the resulting compound is treated with water to afford the acetic acid product. It is important to note that a metal carbonyl complex must be used as a catalyst for step 2 of this process.
Other Methods of Preparing Acetic Acid
Some naphthalene salts of cobalt, chromium, and manganese can be employed as metal catalysts in the oxidation of acetaldehyde. The chemical equation for this reaction can be written as:
O2 + 2CH3CHO → 2CH3COOH
Ethylene (C2H4) can be oxidized into acetic acid with the help of a palladium catalyst and a heteropoly acid, as described by the following chemical reaction.
O2 + C2H4 → CH3COOH
Some anaerobic bacteria have the ability to directly convert sugar into acetic acid.
C6H12O6 → 3CH3COOH
It can be noted that no ethanol intermediates are formed in the anaerobic fermentation of sugar by these bacteria.
Physical Properties of Acetic Acid
Even though ethanoic acid is considered to be a weak acid, in its concentrated form, it possesses strong corrosive powers and can even attack the human skin if exposed to it. Some general properties of acetic acid are listed below.
- Ethanoic acid appears to be a colourless liquid and has a pungent smell.
- At STP, the melting and boiling points of ethanoic acid are 289K and 391K respectively.
- The molar mass of acetic acid is 60.052 g/mol and its density in the liquid form is 1.049 g.cm-3.
- The carboxyl functional group in ethanoic acid can cause ionization of the compound, given by the reaction: CH3COOH ⇌ CH3COO– + H+
- The release of the proton, described by the equilibrium reaction above, is the root cause of the acidic quality of acetic acid.
- The acid dissociation constant (pKa) of ethanoic acid in a solution of water is 4.76.
- The conjugate base of acetic acid is acetate, given by CH3COO–.
- The pH of an ethanoic acid solution of 1.0M concentration is 2.4, which implies that it does not dissociate completely.
- In its liquid form, acetic acid is a polar, protic solvent, with a dielectric constant of 6.2.
The metabolism of carbohydrates and fats in many animals is centered around the binding of acetic acid to coenzyme A. Generally, this compound is produced via the reaction between methanol and carbon monoxide (carbonylation of methanol).
Chemical Properties of Acetic Acid
The chemical reactions undergone by acetic acid are similar to those of other carboxylic acids. When heated to temperatures above 440oC, this compound undergoes decomposition to yield either methane and carbon dioxide or water and ethenone, as described by the following chemical equations.
CH3COOH + Heat → CO2 + CH4
CH3COOH + Heat → H2C=C=O + H2O
Some metals such as magnesium, zinc, and iron undergo corrosion when exposed to acetic acid. These reactions result in the formation of acetate salts.
2CH3COOH + Mg → Mg(CH3COO)2 (magnesium acetate) + H2
The reaction between ethanoic acid and magnesium results in the formation of magnesium acetate and hydrogen gas, as described by the chemical equation provided above.
Other Reactions
Acetic acid reacts with alkalis and forms acetate salts, as described below.
CH3COOH + KOH → CH3COOK + H2O
This compound also forms acetate salts by reacting with carbonates (along with carbon dioxide and water). Examples of such reactions include:
2CH3COOH + Na2CO3 (sodium carbonate) → 2CH3COONa + CO2 + H2O
CH3COOH + NaHCO3 (sodium bicarbonate) → CH3COONa + CO2 + H2O
The reaction between PCl5 and ethanoic acid results in the formation of ethanoyl chloride.
Uses of Acetic Acid
Ethanoic acid is a very important organic compound in the day-to-day lives of humans. Some important uses of acetic acid are listed below.
- Acetic acid is used as an antiseptic due to its antibacterial qualities
- The manufacture of rayon fiber involves the use of ethanoic acid.
- Medically, acetic acid has been employed to treat cancer by its direct injection into the tumour.
- Being the major constituent of vinegar, it finds use in the pickling of many vegetables.
- The manufacture of rubber involves the use of ethanoic acid. It is also used in the manufacture of various perfumes.
- It is widely used in the production of VAM (vinyl acetate monomer).
- When two molecules of acetic acid undergo a condensation reaction together, the product formed is acetic anhydride.
Acetic Acid as a Solvent
In its liquid state, CH3COOH is a hydrophile (readily dissolves in water) and also a polar, protic solvent. A mixture of acetic acid and water is, in this manner, similar to a mixture of ethanol and water. Acetic acid also forms miscible mixtures with hexane, chloroform, and several oils. However, it does not form miscible mixtures with long-chain alkanes (such as octane).
The desirable solvent properties of acetic acid, along with its ability to form miscible mixtures with both polar and non-polar compounds, make it a very important industrial solvent. It is widely used in the industrial preparation of dimethyl terephthalate (DMT).
Thus, the general properties, uses, and structure of acetic acid are discussed briefly in this article. To learn more about acetic acid and the properties of the carboxyl group found in carboxylic acids, register with BYJU’S and download the mobile application on your smartphone.
Frequently Asked Questions-FAQs
What is acetic acid used for?
The most popular application of acetic acid is its use in vinegar. It is also extremely useful in the production of the vinyl acetate monomer (often abbreviated to VAM). This monomer is an important prerequisite in the production of paints and adhesives.
Is acetic acid a strong acid?
No, CH3COOH is a weak acid. It undergoes complete dissociation only when it is reacted with a strong base. Hydrochloric acid is a much stronger acid than acetic acid.
How can acetic acid be prepared?
It can be prepared by reacting methanol with hydrogen iodide and adding carbon monoxide to the product (methyl iodide) in order to obtain acetyl iodide. Upon hydrolysis, acetyl iodide yields acetic acid.
Is acetic acid a vinegar ?
Vinegar is a solution of acetic acid in water and contains between 5% to 8% ethanoic acid by volume.
Is acetic acid harmful to humans ?
Exposure to more concentrated solutions of acetic acid (>25%) can cause corrosive damage. Breathing vapours with high levels of acetic acid can cause irritation of eyes, nose and throat, cough, chest tightness, headache, fever and confusion

Comments