What is Magnesium Sulfate (MgSO4)?
MgSO4 is an inorganic salt with a chemical name Magnesium sulfate. It is also known as Sulfuric acid magnesium salt or Magnesium sulfate anhydrous. It is commonly referred to as Epsom salt. Magnesium sulfate is a crystalline solid that has a white appearance and is odourless. It is majorly used as a bath soak to get rid of sore muscles, to ease the pain of sprains and bruises, etc. It also helps in the removal of splinters and is quite effective.
A magnesium salt with sulphate is known as magnesium sulphate. It functions as a calcium channel blocker, anaesthetic, tocolytic, anti-arrhythmia, analgesic, fertiliser, anticonvulsant, cardiovascular medication, analgesic, and all of the above. It consists of a metal sulphate, an organic magnesium salt, and a magnesium salt.
Table of Contents
- Properties of Magnesium sulfate-MgSO4
- Structure of MgSO4 Molecule
- Uses of Magnesium Sulfate
- Health Hazards Associated with Magnesium Sulfate
- Frequently Asked Questions – FAQs
Properties of Magnesium sulfate – MgSO4
| MgSO4 | Magnesium sulfate |
| Molecular Weight/ Molar Mass | 120.366 g/mol |
| Density | Anhydrous: 2.66 g/cm3 |
| Taste | Bitter taste, Saline |
| Melting Point | 1,124 °C |
It is important to note that anhydrous magnesium sulfate undergoes decomposition at temperatures above 1124 degrees Celsius. Since MgSO4 is an ionic salt, it exhibits high solubility in water. It can also be noted that the solubility of magnesium sulfate in water increases when the temperature is increased. For example, the solubility of anhydrous MgSO4 in water at a temperature of OoC is 269 grams per litre. When the temperature is increased to 100oC, the solubility of this ionic salt in water almost doubles to 502 grams per litre.
Magnesium sulfate is known to be a component of many double salts (ionic salts containing more than one cation or more than one anion). Commonly known double salts containing MgSO4 include potassium magnesium sulfates and sodium magnesium sulfates. Under standard conditions for temperature and pressure (STP), MgSO4 exists as a white crystalline solid which does not have any characteristic odour.
Structure of MgSO4 Molecules
The structure of a magnesium sulfate molecule is illustrated below. Note that MgSO4 molecules contain one Mg2+ cation (the magnesium ion) and one SO42- anion (the sulfate anion).
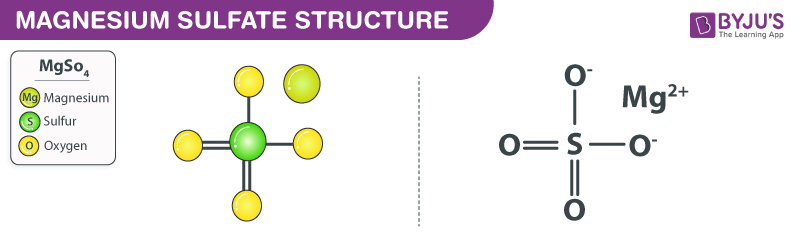
An ionic bond is formed between the magnesium cation and the sulfate anion in magnesium sulfate. In the sulfate anion, there exist two sulphur-oxygen double bonds and two sulphur-oxygen single bonds. The oxygen atoms that are singly bonded to the sulphur atom each hold a negative charge of magnitude -1. Therefore, the total charge on the sulphate anion is -2. The charge on the magnesium cation has a magnitude of +2. Therefore, the positive and negative charges in this ionic compound cancel each other out, resulting in a neutrally charged magnesium sulphate molecule. However, when this compound is dissolved in water and other polar solvents, the magnesium and the sulphate ions dissociate.
Uses of Magnesium Sulfate
- It is used in agriculture to increase the magnesium and sulphur content in the soil.
- It is used in making beer as a brewing salt.
- It is used as a coagulant to make tofu.
- It is used in organic synthesis as a desiccant.
- Consumption of magnesium sulfate orally as osmotic purgative or saline laxative.
- Medically it is used in IV (intravenously) to control seizures in pregnant women.
One of the most important applications of magnesium sulfate is in the preparation of intravenous magnesium, a vital medication for the treatment of eclampsia. It is also used in replacement therapies for the treatment of magnesium deficiency. It can also be noted that magnesium sulfate can be employed as a coagulating agent for the preparation of certain food items like tofu.
Health Hazards Associated with Magnesium Sulfate
This compound is dangerous when used in a higher dose than that is recommended by your doctor or instructed on the package. A high dosage of this compound causes serious side effects and can also be life-threatening. Abnormally high concentrations of magnesium in plasma can result in a medical condition known as hypermagnesemia.
The chemical causes only a little eye and respiratory tract irritation. Particularly if powdered, airborne particles can quickly accumulate to a dangerous concentration. Epsom salt and bitter salt are other names for magnesium sulphate heptahydrate.
Frequently Asked Questions
Is magnesium sulfate a compound or mixture?
Magnesium sulfate, MgSO4, is a colourless crystalline substance formed by the sulphur dioxide and air reaction of magnesium hydroxide.
Is magnesium sulfate soluble in alcohol?
Magnesium sulfate heptahydrate is a white, crystalline, or brilliant, usually needle-like crystals. It is readily soluble in water, in boiling water more easily soluble, and in alcohol virtually insoluble.
What is the hydrating formula for MgSO4
To obtain the mole ratio, divide the moles of water by moles of anhydrate. 5 H2O/1 mole MgSO4 moles = 5:1. To write the equation, use the mole ratio. Because for every 1 mole of MgSO4 there are 5 moles of H2O, the formula is 5H2O MgSO4.
What is magnesium sulfate heptahydrate used for?
One of the main applications of magnesium sulfate is to be used as a fertilizer in farming and gardening. For bath salts, magnesium sulfate is used.
How moles are calculated?
Use the molecular formula to calculate the molar mass; divide the weight of the compound by the compound’s molar mass measured in grams to obtain the number of moles.
To explore more about the structure, physical and chemical properties of Magnesium sulfate (MgSO4) from the experts, register with BYJU’S now!
Other related links:
| Sulfur | Magnesium |


Comments