Table of Contents
What is Calcium Chloride (CaCl2)?
CaCl2 is an ionic compound with chemical name Calcium Chloride. It is also called Calcium chloride anhydrous or Calcium dichloride.
It is an ionic compound of chlorine and calcium. At room temperature, it is a crystalline solid white in colour. It is highly soluble in water and hence is hygroscopic in nature. It is odourless and has a very high enthalpy change of solution. This compound is widely used for dust control and de-icing. It is prepared as by the following –
-
-
-
- By reacting calcium carbonate and hydrochloric acid or
- Directly from limestone, but a large amount is also produced as a by-product of the Solvay process.
-
-
Calcium chloride was discovered in the 15th century but received little attention or study until the latter part of the 18th century. All of the early work was done with laboratory-prepared samples since it was not produced on a commercial scale until after the ammonia-soda process for the manufacture of soda ash was in operation. It was actually considered a waste product until its uses were discovered.
Properties of Calcium Chloride – CaCl2
| CaCl2 | Calcium Chloride |
| Molecular Weight/ Molar Mass | 110.98 g/mol |
| Density | Anhydrous: 2.15 g/cm3 |
| Boiling Point | 1,935 °C |
| Melting Point | 772 °C |
Calcium Chloride structure (CaCl2 Structure)
Calcium chloride molecules feature two ionic bonds between the single calcium cation and the two chloride anions. The structure of calcium chloride molecules is illustrated below. It can be noted that the calcium cation holds a charge of magnitude +2 and each chloride anion holds a charge of magnitude -1. The compound is, therefore, electrically neutral.
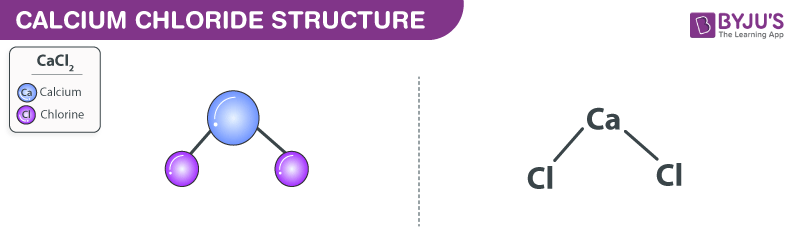
Structure of Calcium Chloride Molecules
Preparation of Calcium Chloride
The steps listed below can be followed in order to prepare calcium chloride:
Step 1:
Take a beaker. Wear gloves and place limestones in it until the beaker is filled up by a quarter of its total volume.
Step 2:
Add approximately 1/4th of a beaker of HCl (hydrochloric acid) to the limestones.
Step 3:
As the HCl dissolves the limestone it starts to bubble. Mix the contents in the beaker gently and take care that the reaction completes. Add a little limestone if all the limestones dissolve in it completely.
Step 4:
Filter off the solids by pouring the solution through the filter paper as soon as the solution stops bubbling.
Step 5:
Heat the second beaker which contains the calcium chloride solution. Solid calcium chloride is the solid left after the water evaporates.
Calcium Chloride Solutions
Calcium chloride is highly soluble in water, because of its high solubility it is used to obtain solutions having relatively high densities. For example, densities as high as 1430kg/m3 are achieved at 208oC and 1570kg/m3 at 808oC. The oil and gas drilling industries frequently exploit these high densities when completing or reworking wells. Density or specific gravity can also be used to determine the molal concentration, c of calcium chloride in water.
The densities of calcium chloride solution at various values and different temperatures have been identified. Densities and apparent molar volumes of aqueous calcium chloride solutions at temperatures from 323K to 600K and at pressure up to 40MPa have also been reported. Viscosity is an important property of calcium chloride solutions in terms of engineering design and in application of such solutions to flow through porous media. Data and equations for estimating viscosity of calcium chloride solutions over the temperature range of 20-508oC are available.
Numerous studies on the thermodynamics of calcium chloride solutions were published in the 1980s. Many of these were oriented toward verifying and expanding the Pitzer equations for determination of activity coefficients and other parameters in electrolyte solutions of high ionic strength. Calcium chloride is produced in commercial amounts using many procedures: refining of natural brines, reaction of calcium hydroxide with ammonium chloride in Solvay soda ash production and reaction of hydrochloric acid with calcium carbonate.
A property possessed by some substances of absorbing moisture from the air on exposure. Anhydrous calcium chloride, which possesses this property, is widely used as a drying agent.
Calcium Chloride (CaCl2 ) Uses
-
-
-
- It is used to prevent ice formation and therefore used in deicing.
- Used in the production of activated charcoal.
- Used as a sterilant for male animals.
- It is used in heating pads and self-heating cans.
- It is used to correct the mineral deficiencies in brewing beer
- Calcium chloride is used as an electrolyte in sports drinks
- In laboratories, the drying tubes are usually packed with calcium chloride.
-
-
Health Hazards
-
-
-
- This compound is irritating and needs to be handled with gloves.
- It is relatively safe to handle, but if ingested, it reacts exothermically with water and can cause burning of the mouth or esophagus.
- Calcium chloride is also recommended for the treatment of acute hyperkalemia, hypermagnesemia, and calcium-channel blocker overdose.
-
-
Frequently Asked Questions
What is calcium chloride made of?
Calcium chloride is a calcium-derived salt that occurs naturally. It is a solid white and can be rendered synthetically as well.
Is calcium chloride a natural product?
Natural calcium chloride contains small quantities of sodium chloride and potassium chloride transported from the natural feedstock of the brine. This covers nearly all food-grade calcium chloride applications.
What is calcium chloride commonly used for?
Calcium chloride is an excellent desiccant as a hygroscopic agent to eliminate dissolved moisture in liquids and is suitable for use in food packaging to improve dryness and avoid spoilage.
What happens when calcium chloride is exposed to air?
Because calcium carbide is a fragile material, when exposed to air, it absorbs water from the atmosphere. When anhydrous calcium chloride becomes released in the sun, it also absorbs heat from the atmosphere and becomes a colourless solution.
Is calcium electrically conductive?
Calcium is more difficult than lead, but with an effort, it can be cut with a knife. Although calcium is a weaker electricity conductor than copper or aluminium by weight, due to its very low density, it is a better mass conductor than both.
Why is calcium chloride used in drinking water?
It’s commonly used in sports drinks and other beverages, including bottled water, as an electrolyte. Calcium chloride’s highly salty taste is used to flavour pickles, without increasing the sodium content of the food.
What foods contain calcium chloride?
Calcium chloride is used as a firming agent in canned vegetables, to firm soybean curds into tofu, and to make a caviar substitute from vegetable or fruit juices. It’s commonly used in sports drinks and other beverages, including bottled water, as an electrolyte.
What is bad about calcium chloride?
Calcium chloride poses some serious dangers to your health and safety. Calcium chloride can cause burns in the mouth and throat, excess thirst, vomiting, stomach pain, low blood pressure, and other potential serious health effects if ingested. This may also irritate the skin, causing prolonged dryness or damp skin to dry out.
To explore more about the structure, the physical and chemical properties of CaCl2 from the experts register to BYJU’S now!
Other related links:
| Calcium | Chlorine |


Comments