What is Potassium Bicarbonate?
Potassium bicarbonate is a chemical compound with the formula KHCO3. It is made up of a potassium cation (K+) and a bicarbonate anion (HCO3–).
The IUPAC name of this compound is potassium hydrogen carbonate. Under standard conditions, potassium bicarbonate exists as a white solid.

Potassium Bicarbonate
KHCO3 is a monopotassium salt of H2CO3 (carbonic acid). Similar to sodium bicarbonate (baking soda), potassium bicarbonate is alkaline in nature. It is widely used as an antacid since it has the ability to neutralize gastric acid.
Potassium Bicarbonate Structure
Potassium bicarbonate molecules feature an ionic bond between K+ and HCO3– ions. The structure of a KHCO3 molecule is illustrated below.
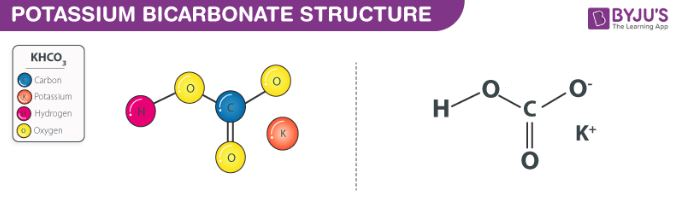
The bicarbonate ion is formed when carbonic acid undergoes deprotonation. This polyatomic ion consists of a central carbon atom which is chemically bonded to three oxygen atoms. One of these oxygen atoms is bonded to a hydrogen atom. The negative charge of magnitude -1 is delocalized through the remaining oxygen atoms due to resonance.
Potassium Bicarbonate Preparation
When an aqueous solution of K2CO3 (potassium carbonate) is treated with carbon dioxide gas, KHCO3 is formed. The chemical equation for this reaction is given by:
CO2 + K2CO3 + H2O → 2KHCO3
The standard enthalpy of formation for this compound corresponds to -963.2 kilojoules per mole. When heated to 120oC, potassium bicarbonate begins to undergo a decomposition reaction to yield water, carbon dioxide, and potassium carbonate.
Properties of KHCO3
1. Chemical Data
| Potassium Bicarbonate | KHCO3 |
| Molar Mass | 100.115 grams per mole |
| Density | 2.17 grams per cubic centimeter |
| Melting Point | 292oC (Starts to Decompose at 100oC) |
| Boiling Point | Decomposes |
2. Physical Properties
- At room temperature, KHCO3 exists in the form of white crystals.
- This compound does not have any distinct odour and is considered to be odourless.
- At a temperature of 20oC, potassium bicarbonate has a solubility of 22.4g/100mL in water.
- The acidity of this compound corresponds to 10.329.
3. Chemical Properties
- When reacted with acids, this compound forms a potassium salt with the acid. For example, its reaction with hydrochloric acid yields potassium chloride.
- The chemical equation for this reaction is: HCl + KHCO3 → KCl + CO2 + H2O
- This compound is insoluble in alcohols and most other organic solvents.
What are the Uses of Potassium Bicarbonate?
Potassium bicarbonate is widely used in organic farming in order to control powdery mildew. Some other applications of this compound are listed below.
- This compound is used as a leavening agent in baking processes since it can liberate carbon dioxide.
- Several dry chemical fire extinguishers use KHCO3 as a component.
- Since it is inexpensive, non-toxic, and alkaline, potassium bicarbonate has several applications in pH regulation.
- It is used as a buffering agent to control the pH of several medications.
- It is also added as an additive in the process of winemaking since it helps regulate the pH.
- In order to improve the taste of club soda, KHCO3 can be added to it.
- This compound can also be used to neutralize acidic soils to promote agriculture.
Frequently Asked Questions – FAQs
What are the uses of potassium bicarbonate?
Potassium is a mineral that is contained naturally in food, and is important for many of the body’s normal functions, particularly beating of the heart. Potassium bicarbonate is used to prevent or treat a deficit of potassium (hypokalaemia).
Is potassium bicarbonate the same as baking soda?
No, baking soda is made up of sodium bicarbonate. However, potassium bicarbonate can be used as an alternative to baking soda because it has the same leavening capabilities, but it does not contain any sodium.
Is potassium bicarbonate an antacid?
Potassium bicarbonate can be used as an antacid, electrolyte replenisher and as a supplement for potassium. It can also be used in medication formulations as an excipient. The antacid potential of potassium bicarbonate is achieved by neutralizing hydrochloric acid by increasing the gastrointestinal pH.
Is KHCO3 ionic or covalent?
The ions K+ and HCO3– form an ionic bond in potassium bicarbonate molecules. A KHCO3 molecule’s structure is depicted below. When carbonic acid is deprotonated, the bicarbonate ion is formed. A central carbon atom is chemically bound to three oxygen atoms in this polyatomic ion.
To learn more about potassium bicarbonate and other potassium compounds (such as potassium permanganate), register with BYJU’S and download the mobile application on your smartphone.

Comments