What is Nitric Acid?
Nitric Acid is a inorganic compound with chemical formula HNO3.
It is a highly corrosive mineral acid, fuming, colourless and plays a important role in the manufacture of fertilisers and explosives.
It is also known as the spirit of niter and aqua fortis. In its pure form, it is colourless but as it gets older it turns into a yellow cast. This colour appears due to the decomposition of Nitric acid to oxides of nitrogen and water. It is highly corrosive and toxic. It causes severe skin burn. It reacts with hydroxides, metals, and oxides to form nitrate salts.
HNO3 is used as a strong oxidizing agent. It can be manufactured by the catalytic oxidation of ammonia. It is a common reagent used in laboratories and an important chemical used in industries to manufacture explosives and fertilizers. The PH of Nitric acid is approximately 3.01.
Table of Contents |
Structure of HNO3 Molecules
Nitric acid molecules contain 3 oxygen atoms, 1 nitrogen atom, and 1 hydrogen atom. In HNO3 molecules, one of the oxygen atoms is doubly bonded to the central nitrogen atom. Another oxygen atom is singly bonded to the central nitrogen atom and also singly bonded to a hydrogen atom. The last oxygen atom in the nitric acid molecule has a charge of -1 and is singly bonded to the central nitrogen atom. Since the nitrogen atom at the center of the molecule is participating in four covalent bonds (with 3 oxygen atoms), it has a charge of +1.
Therefore, the net charge on the nitric acid molecule is 0 (the positive charge on the nitrogen atom and the negative charge on the oxygen atom cancel each other out). It can be noted that the charges in these molecules can be delocalized due to resonance. The structure of nitric acid molecules is illustrated below.
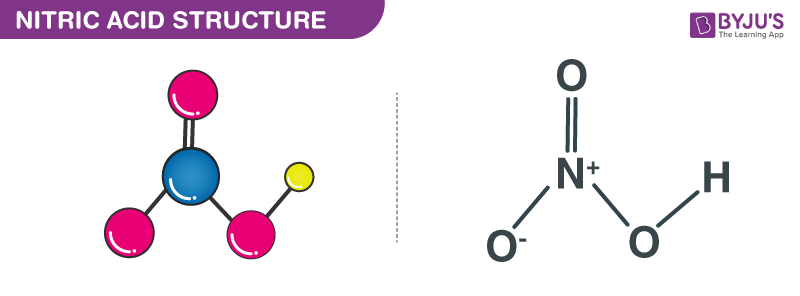
Structure of Nitric Acid
Laboratory Preparation of Nitric Acid – HNO3
Principle
A more volatile acid can be displaced from its salt by a less volatile acid. This is the basic principle in the laboratory preparation of nitric acid.
Illustration
Nitric acid is a more volatile acid than sulphuric acid is displaced by sulphuric acid from metal nitrates.
Reactants
50gm of potassium nitrate (KNO3) + 25ml of concentrated sulphuric acid (H2SO4) is taken in a round bottom flask. The reactants are heated to about 200°C taking care that the temperature does not cross 200°C.
The reaction
KNO3 + H2SO4 → KHSO4 + HNO3
(Salt of more volatile acid + less volatile acid → displaces more volatile acid)
Apparatus Setup

Laboratory Preparation of Nitric Acid – HNO3
Method of Collection
The vapours of nitric acid are cooled and condensed for collection as shown in the diagram.
Physical Properties of Nitric Acid – HNO3
| HNO3 | Nitric Acid |
| Molecular Weight/ Molar Mass | 63.01 g/mol |
| Density | 1.51 g/cm³ |
| Boiling Point | 83 °C |
| Melting Point | -42 °C |
Chemical Properties of Nitric Acid – HNO3
-
-
-
- Nitric acid is a very strong acid, turns blue litmus red.
- Nitric acid decomposes on standing to form brown nitrogen dioxide. This is the reason why it becomes brownish over time though fresh nitric acid is colourless.
4HNO3 → 4NO2 + O2 + 2H2O - Nitric acid liberates hydrogen gas with metals above hydrogen in the metal activity series.
Mg + 2HNO3 → Mg(NO3)2 + H2
Mn + 2HNO3 → Mn(NO3)2 + H2
-
-
Uses of Nitric Acid
-
-
-
- It is used to produce ammonium nitrates to manufacture plastic, dye, and fertilizers
- It is used in making explosives such as TNT
- It is used in liquid-fueled rockets as an oxidizer
- In its pure form, it is used in the removal of the wart
- It is used as a chemical doping agent in electrochemistry
-
-
Recommended Videos

Further Reading
| Uses of Nitric Acid | Chemical Reactions |
| Uses of Phosphoric Acid | Oxyacids and Ammonia |
Learn more about the chemical behaviour and importance of HNO3 from the expert faculties at BYJU’S – India’s largest education company.
Frequently Asked Questions on Nitric Acid
How do you identify nitric acid?
Nitric acid is a liquid that is colorless to yellow with a strong, shocky, acidic odor. Concentrated nitric acid emit nitrogen dioxide and nitrogen oxide gases 85-100 percent).
What does nitric acid taste like?
Nitric acid is an acid that has acidic properties. Such properties include a pH of less than 7, a bitter taste, and sometimes a violent reaction to other metals. The chemical formula for nitric acids is HNO3. Nitric acid is a white, very corrosive, colorless liquid.
Does nitric acid react with water?
H2 acts as a basis for abstracting an H+ from nitric acid. The resulting H3O+, hydronium, is the conjugate acid, while the base of the conjugate is the NO3–, nitrate (this is the nitric acid molecule, but its H+ is removed.
What metals will nitric acid dissolve?
Some metals and alloys are oxidized by nitric acid; however, when struck by concentrated nitric acid, gold and platinum are not oxidized and certain metals are passivated. By using a mixture of acids or a dilute nitric solution, these metals can be dissolved.
What neutralizes nitric acid?
Sodium bicarbonate neutralizes Nitric acid. If this is accurate, neutralizing one part of nitric acid requires one part of sodium bicarbonate. Perhaps 5 percent nitric is not much heavier than water, so 100 gallons of the solution equals 834 pounds of nitric acid equals 42 pounds.


Comments