Ethanol formula is given here along with its structure. Ethanol, also known as ethyl alcohol is simple alcohol and is generally found in alcoholic beverages. Ethyl alcohol or ethanol is also represented as EtOH. The formula for ethanol and its structure is simple and is mentioned below with explanations.
Table of Contents
What is the Formula for Ethyl Alcohol or Ethanol?
The formula for ethyl alcohol or ethanol is C2H5OH or CH3CH2OH.
Ethanol is a compound of carbon, hydrogen and oxygen elements was described by Antoine Lavoisier and its chemical formula was determined by Nicolas-Theodore de Saussure in 1808. Five years later, Archibald Scott Couper published the structural formula of ethanol.
Ethyl alcohol consists of two carbon chains, i.e. ethane which has a hydroxyl group (-OH) group attached to it. The proper ethyl alcohol (ethanol) chemical formula and structural formula re given in the following points.
Recommended Videos

Ethyl Alcohol- Chemical Formula
Ethanol has 2 carbon atoms, 5 hydrogen atoms, and an OH group. The chemical formula of ethanol is given by-
| Chemical Formula for Ethanol (Ethyl Alcohol) | C2H5OH |
| Formula for Ethyl Alcohol | CH3CH2OH |
Ethyl alcohol is a colourless, volatile liquid with a characteristic odour and a pungent taste. It has a flashpoint of 55°F, is classified as a depressant drug, and is toxic when ingested in large quantities.
The molecular formula describes the composition of ethanol molecules two carbon atoms, six hydrogen atoms and one atom of oxygen occur per molecule but gives us no structural information. Structural information is how the atoms are connected and how the molecule fills the space.
Ethyl Alcohol Structural Formula
The carbon atoms in an ethanol molecule are sp3 hybridized, i.e. they have a free rotation. It is the second most simple alcohol and is represented as-
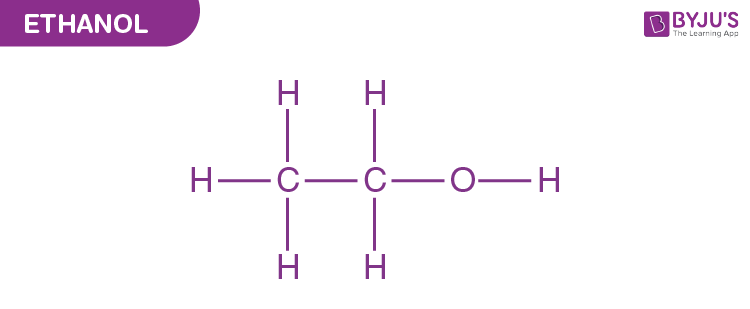 There are various uses of ethanol and the most common one is in the pharmaceutical industry where it is used as an antiseptic. Apart from that, ethyl alcohol is also used in cosmetics, and in biotechnological industries. Click uses of methanol and ethanol to learn more about its uses in everyday life.
There are various uses of ethanol and the most common one is in the pharmaceutical industry where it is used as an antiseptic. Apart from that, ethyl alcohol is also used in cosmetics, and in biotechnological industries. Click uses of methanol and ethanol to learn more about its uses in everyday life.
Uses of Ethanol
-
-
-
- Ethanol is used in alcoholic beverages, solvents, scents, flavourings, colouring, medicines, chemical synthesis, and thermometers.
- Ethanol is used as a fuel, an excellent solvent and an important raw material for making other chemicals.
Pure ethanol is a toxic liquid, methanol which is even more toxic, is sometimes added to it to put off anyone wanting to drink it. - A mixture of ethanol and methanol is known as methylated spirits or sometimes ‘meths’ used in camping stoves.
- Used as a raw material for making esters which in turn are used as solvents in food flavouring, and in the manufacture of cosmetics.
- Vinegar, a weak solution of ethanoic acid, is produced from ethanol by a biochemical process. It involves oxidation caused by bacteria called acetobacter.
-
-
Frequently Asked Questions – FAQs
Who discovered ethyl alcohol?
Antoine Lavoisier described ethanol as a carbon, hydrogen and oxygen compound, and in 1808 Nicolas-Théodore de Saussure determined the chemical formula for ethanol, and in 1858 Archibald Scott Couper released a structural formula for ethanol.
Why is ethyl alcohol flammable?
Ethanol is an alcohol created by an ethene-water mixture. Ethanol is flammable, as is the case with all carbohydrates, it will mix relatively well with oxygen to release carbon dioxide and water as waste products.
Why is ethanol a good solvent?
Due to its hydroxyl (OH) group, ethanol is a very polar molecule with elevated oxygen electronegativity allowing for hydrogen bonding with other molecules. Ethanol can, therefore, function as a useful solvent and dissolve polar as well as non-polar substances.
What is the percentage of ethyl alcohol?
A combination of 95% ethyl alcohol and 5% water is the ethyl alcohol used in fuels and nearly all industrial activities. Both, absolute and 95% ethyl alcohol are extremely toxic.
What is the melting point of ethyl alcohol?
The melting point of ethyl alcohol is -114.1 °C
Stay tuned with BYJU’S to get the chemical formulas of different other compounds and get complete assistance for the exams.


Comments