What is Williamson Ether Synthesis?
The general method for the synthesis of ether is Williamson ether synthesis, involves nucleophilic displacement of a halide ion or other good leaving group by an alkoxide ion.
The name of the reaction was coined after Alexander William Williamson developed it in 1850. Williamson Ether Synthesis is a reaction that uses deprotonated alcohol and an organohalide to form an ether.
- Williamson Ether Synthesis usually takes place as an SN2 reaction of a primary alkyl halide with an alkoxide ion. The structure of ethers was proved due to this chemical reaction.
- SN2 pathway is required for the synthesis this reaction is useful only when the alkyl halide is primary or secondary.
- The Ethers produced in this way have more carbon atoms than either of the starting materials and thus are more complex structures.
Thus, Organic chemistry’s history holds a special place for the reaction. Read Williamson Ether Synthesis and its uses.
The basic mechanism of the reaction is:
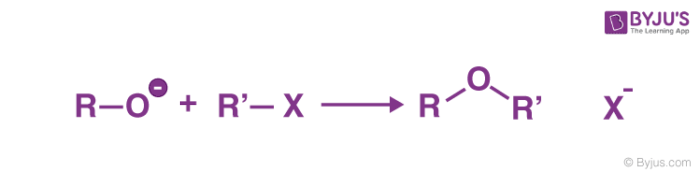
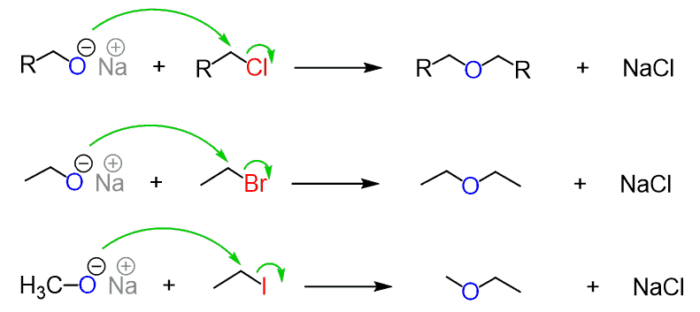
Diethyl Ether and Sodium Chloride are formed when Sodium Ethoxide and Chloroethane react. The reaction is displayed below.
Na+C2H5O− + C2H5Cl → C2H5OC2H5 + Na+Cl−
For example, consider the following Williamson Ether Synthesis reaction.
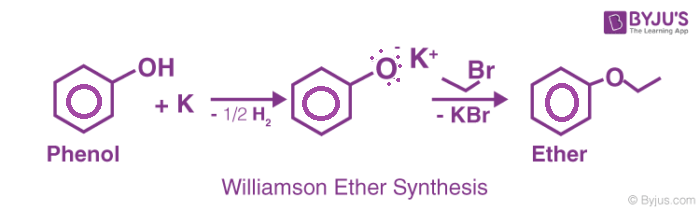
Alkoxide ions are good nucleophiles and displace halide ions from alkyl halides resulting in the formation of a new carbon oxygen bond. Alkoxides are produced by treatment of alcohols with either a base or an alkali metal.
Mechanism of the Reaction
- The nucleophile attacks the alkyl halide forming an ether from the back.
- This response takes place in a single step, which is both cleavage and bond formation.
- If halides are sterically impeded then alkoxide acts as a basis and protons in β-place are accessible.
- The products derived from a response to elimination.
Uses of Ether
- The preparation of ethers in labs and industrially is mostly done through this process. Symmetrical and asymmetrical both forms of ethers are simply prepared.
- Two choices of reactants are available which is finally agreed upon depending on the reactivity and availability.
- Two alcohols are also used to produce ethers by Williamson reaction. The two are reacted together after one of them is transformed a leaving group (tosylate).
- The alkylating agent is preferred to be primary whereas the alkoxide could be primary secondary or tertiary. If not a Halide, a sulfonate ester created for the purpose of the reaction are the leaving group.
Conditions Required
- In situ preparation of alkoxide ions is done as they are extremely reactive.
- Potassium hydroxide or a carbonate base is used for the laboratory preparation, whereas phase transfer catalysis is used when doing industrial synthesis.
- Acetonitrile and N, N-dimethylformamide are used as solvents.
- It takes around 1-8 hours to complete the reaction and it takes place at a temperature of around 50-100 °C.
- One can get a yield of between 50-95% in the lab preparation as using up the raw material completely is rare, due to side reactions.
- The industrial procedure shows better quantitative results.
- Lab synthesis does not usually require a catalyst but if the alkylating agent is unreactive then to improve the rate of reaction iodide salt can be added which yields an extremely reactive iodide after a halide exchange with the chloride.
- Silver salts like Silver Oxide are used in extreme cases which helps the leaving halide group and makes its exodus more simple.
Limitations of the Reaction
There are few limitations of Williamson Ether Synthesis.
- Tertiary alkyl halides or sterically hindered primary or secondary alkyl halides tend to undergo E2 elimination in the presence of the alkoxide that in addition to being a nucleophile also act as a base.
- Alkali phenoxides may undergo C-alkylation in addition to expected O-alkylation.
- This process for preparing ethers is too limited to be of any practical value for synthetic organic chemists.
Recommended Video
Alcohols Phenols and Ethers

Frequently Asked Questions – FAQs
What is Williamson synthesis given as an example?
Williamson’s synthesis: It is used for both basic and mixed ether preparation. The alkyl halide is heated to form corresponding ethers with alcoholic sodium or potassium alkoxide. So, methyl iodide forms dimethyl ether when heated with alcoholic sodium methoxide.
What are the limitations of Williamson synthesis?
Williamson Ether Synthesis presents few limitations. Tertiary alkyl halides or primary or secondary alkyl halides that are sterically impeded continue to undergo E2 removal in the presence of alkoxide, which serves as a base in addition to being a nucleophile.
Why is Williamson ether synthesis important?
The synthesis of the Williamson ether is an organic reaction which forms an ether from an organohalide and a deprotonated alcohol (alkoxide). This reaction is significant in the organic chemistry history as it has helped to prove the ethers structure.
Is Williamson ether synthesis sn1 or sn2?
Alkyl halides (or tosylates) react to ethers by forming alkoxy ions. This reaction is called the synthesis of the ether. Examples: Note: Because this is an SN2 reaction and goes through a backside attack, the carbon configuration will be reversed (note the last two examples).
What was ether used for?
Ether was used in the history of medicine, particularly as a remedy for illnesses such as scurvy or pulmonary inflammation, until its creation as a surgical anaesthetic. Ether, a pleasant-smelling, colourless and highly flammable liquid, may be vaporized into a gas that reduces pain but keeps patients conscious.
Learn more about the organic reactions and its mechanism from the expert faculties at BYJU’S.


Comments