What is Carbonic Acid?
Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. Solutions of carbon dioxide in water contain small amounts of this compound. Its chemical formula can also be written as OC(OH)2 since there exists one carbon-oxygen double bond in this compound.
Carbonic acid is often described as a respiratory acid since it is the only acid that is exhaled in the gaseous state by the human lungs. It is a weak acid and it forms carbonate and bicarbonate salts.
H2CO3 can dissolve limestone, which leads to the formation of calcium bicarbonate (Ca(HCO3)2. This is the reason for many features of limestone, such as stalagmites and stalactites.
Table of Content
- Carbonic Acid Structure
- Recommended Videos
- Preparations of Carbonic acid
- Properties of Carbonic acid-H2CO3
- Uses of Carbonic acid-H2CO3
- Importance of Carbonic Acid in Blood
- FAQs
-
Carbonic Acid Structure
The structure of carbonic acid is illustrated below.
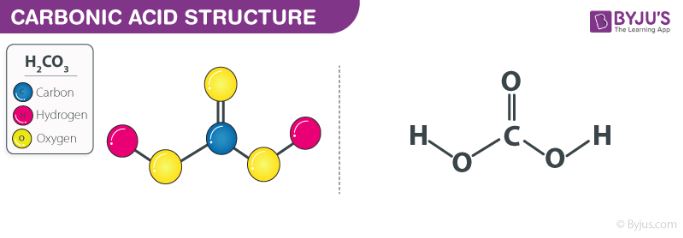
Carbonic Acid Structure
Recommended Videos

Preparations of Carbonic acid-H2CO3
From the illustration provided above, it can be understood that the structure of carbonic acid consists of one carbon-oxygen double bond and two carbon-oxygen single bonds. The oxygen atoms participating in a single bond with the carbon each have one hydrogen atom attached to them.
Carbonic acid, which is formed by the dissolution and hydrolysis of CO2 in water, is the major natural leaching agent in many temperate ecosystems. Carbonic acid is both weak and unstable and quickly dissociates into hydrogen ions (H+) and bicarbonate ions (HCO3–)
Carbon dioxide, when dissolved in water, participates in the following chemical equilibrium:
CO2 + H2O ⇌ H2CO3
However, only a small amount of carbon dioxide is converted into carbonic acid in the chemical equilibrium described above.
Properties of Carbonic acid-H2CO3
Some important physical and chemical properties of carbonic acid are listed in this subsection.
1. Physical Properties
- The molar mass of carbonic acid is 62.024 grams per mole.
- Its density in its standard state is 1.668 grams per cubic centimetre.
- The compound H2CO3 has a pKa value of 6.35.
- The conjugate base corresponding to carbonic acid is the bicarbonate.
- This compound generally exists as a solution. However, it has been reported that solid H2CO3 samples have been prepared by NASA scientists.
2. Chemical Properties
- H2CO3 is a weak acid and is unstable in nature.
- It undergoes partial dissociation in the presence of water to yield H+ and HCO3– (bicarbonate) ions.
- Carbonic acid is a diprotic acid, and can hence form two types of salts, namely bicarbonates and carbonates.
- Addition of a small quantity of a base to H2CO3 yields bicarbonate salts whereas addition of a base in excess yields carbonate salts.
It can be noted that carbonic acid can be obtained as a by-product of industrial fermentation processes or the burning of fossil fuels at an industrial scale.
Uses of Carbonic Acid
H2CO3 is a very important compound with a wide range of applications. Some of these uses of carbonic acid are listed below.
- The preparation of carbonated water, sparkling wine, and other aerated drinks involve the use of carbonic acid.
- H2CO3 is used in the precipitation of many ammonium salts such as ammonium persulfate.
- It helps in the transportation of carbon dioxide out of the body.
- Various bases containing nitrogen in blood serum are protonated by H2CO3
- Ringworm and other dermatitides are treated via the application of carbonic acid over the affected area.
- Solutions containing this compound are very effective in the cleaning of contact lenses.
- It can be consumed orally in order to induce vomiting whenever required (such as in drug overdose cases).
Importance of Carbonic Acid in Blood
The bicarbonate ion is known to be an intermediate for the transportation of carbon dioxide out of the human body via the process of respiratory gas exchange. The hydration reactions undergone by carbon dioxide are quite slow, especially in the absence of a suitable catalyst. However, the presence of the enzyme family known as carbonic anhydrases in the red blood cells increases the reaction rate. The carbonic anhydrase enzymes work to catalyze the conversion of carbon dioxide and water to the dissociated ions of carbonic acid. This produces bicarbonate anions which get dissolved in the blood plasma. The catalyzed reaction is reversed in the lungs, resulting in the formation of CO2, which is then exhaled.
Importance of Carbonic Acid in Oceans
The absorption of the excess carbon dioxide in the atmosphere (primarily due to human activities) by the oceans is believed to have caused a shift in the pH of the ocean’s water by approximately -0.1. The absorbed carbon dioxide reacts with ocean water and forms H2CO3. This process is commonly referred to as ocean acidification.
Frequently Asked Questions – FAQs
What are the uses of carbonic acid?
Carbonic acid is widely used in the production of soft drinks, artificially carbonated sparkling wines, and other bubbly beverages. Carbonic acid salts are called bicarbonates (or carbonates of hydrogen), and carbonates.
Comment on the acidity of carbonic acid
Carbonic acid is a carboxylic acid that holds a substituted hydroxyl group. It is also a polyprotic acid. This compound is actually diprotic and, therefore, has two protons that dissociate from the primary parent molecule. Therefore, there are two constants of dissociation, of which the first one is for the dissociation into the bicarbonate ion.
What is the role of carbonic acid in blood?
Bicarbonate is an intermediate in the exchange of respiratory gas for conveying CO2 out of the body. In general, the hydration reaction of CO2 is very slow in the absence of a catalyst, but red blood cells contain a substance known as carbonic anhydrase, which increases the reaction rate, creating dissolved bicarbonate (HCO3−) in the blood plasma.
Is carbonic acid a strong acid?
No, carbonic acid is not a strong acid. H2CO3 is a weak acid that dissociates into a proton (H+ cation) and a bicarbonate ion (HCO3- anion). This compound only partly dissociates in aqueous solutions. Furthermore, the conjugate base of carbonic acid, which is the bicarbonate ion, is a relatively good base. These are the reasons why carbonic acid is classified as a weak acid rather than a strong acid.
Is carbonic acid dangerous?
Carbonic acid is not considered to be toxic or dangerous to human health since it is present naturally in the human body. However, it is important to note that exposure to high concentrations of H2CO3 can irritate the respiratory tract and the eyes.
Thus, the structure, physical and chemical properties, and the uses of carbonic acid are discussed. To learn more about this compound and other compounds containing a carbon-oxygen double bond, such as carbonyl compounds, register with BYJU’S and download the mobile application on your smartphone.

Comments