What is Sulphurous Acid?
H2SO3 is a chemical compound with yhe chemical name Sulphurous Acid.
Sulphurous acid is also called Sulphur dioxide solution or dihydrogen trioxosulphate or trioxosulphuric acid. It is an intermediate species for producing acid rain from sulphur dioxide (SO2).
Trioxosulphuric acid is a liquid without colour and has a pungent burning sulphur smell. It is corrosive to tissue and metals. It is a sulphur oxoacid, tautomer of a sulfonic acid, and conjugate acid of a hydrogensulfite.
Table of Contents
- Properties of Sulphurous Acid
- Sulphurous Acid Structure
- Sulphurous Acid Uses
- Sulphurous Acid Reactions
- Sulphurous Acid Health Hazards
- Frequently Asked Questions – FAQs
Properties of Sulphurous Acid – H2SO3
| H2SO3 | Sulphurous acid |
| Molecular weight of H2SO3 | 82.07 g/mol |
| No. of hydrogen bond acceptor | 4 |
| Monoisotopic mass of Sulphurous Acid | 81.97 g/mol |
| No. of hydrogen bond donor | 2 |
Sulphurous Acid Structure – H2SO3
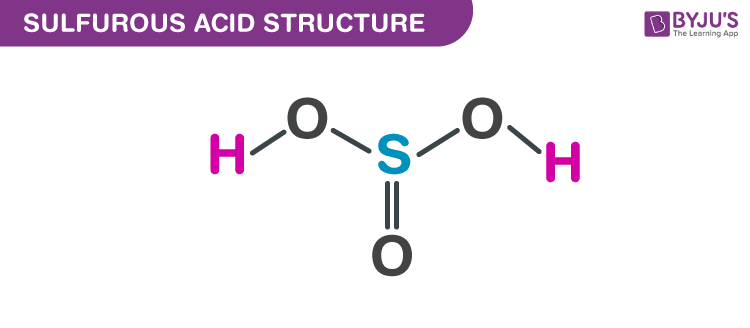
Sulphurous Acid Structure – H2SO3
Sulphurous Acid Uses (H2SO3)
- Sulphurous Acid is used as an intermediate in industries.
- Used as reducing agents.
- Used as disinfectants.
- Used in the manufacturing of paper products.
Sulphurous Acid Reactions
According to Raman spectra of SO2 solutions shows that the intensities of the signals are consistent with the equilibrium as follows:
SO2 + H2O ⇌ HSO−3 + H+
where, Ka = 1.54×10−2 and pKa = 1.81.
Sulphurous Acid Health Hazards
It is a toxic, corrosive, and non-combustible compound. Inhaling, ingesting or skin contact with Sulphur dioxide solution causes severe injury which leads to death. In its molten form, it can cause severe burns to the eyes and skin. Therefore, avoid skin contact with this compound. This compound liberates corrosive, toxic and irritating gases.
Frequently Asked Questions – FAQs
What is sulphurous acid used for?
The sulphurous acid is used in the manufacture of fertilizers, pigments, dyes, drugs, explosives, detergents, and inorganic salts and acids, as well as in petroleum refining and metallurgical processes.
Is sulphurous acid a strong or weak acid?
Unlike sulphuric acid (H2SO4), sulphurous acid (H2SO3) is a weak acid; that is, aqueous sulphurous acid does not dissociate entirely into H+ (H3O+) and bisulfite ions, meaning that the bisulfite ion is comparatively stronger in maintaining a proton when there is a base, such as water.
Is sulphurous acid dangerous?
Sulphuric acid can affect you by breathing in and moving through your skin. Sulfurous acid is a corrosive chemical and
contact can severely irritate and burn the skin and eyes
with possible eye damage.
Is Sulfurous acid a Monoprotic acid?
Sulfurous acid is not a monoprotic acid. It is a diprotic acid, meaning that it yields two protons (H+) per molecule.
Is sulphurous acid soluble?
Sulfuric acid is a colourless oily liquid. It is soluble in water with the release of heat. It is corrosive to metals and tissue.
Learn more about the Structure, physical and chemical properties of H2SO3 from the experts at BYJU’S.

Comments