Table of Content
Elements and compounds are the two forms in which pure substances exist.
Element Definition:
Elements – Elements constitute the simplest chemical substances in which all the atoms are exactly the same.
Compound Definition:
Compounds – Compounds are chemical substances made up of two or more elements that are chemically bound together in a fixed ratio.
Chemistry is the study of the structures, physical properties, and chemical properties of material substances. It is very important to understand that all gases, liquids and solids are not the same. All are different in terms of their composition. This is the reason why the classification of the matter is very important.
Classification of Matter
- Elements
- Compounds
- Mixtures
Here we are going to discuss two categories of the matter: Elements and Compounds
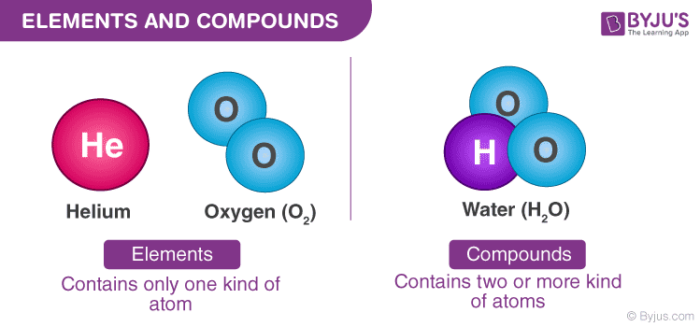
What is a Compound?
When two or more elements chemically combine in a fixed ratio by mass, the obtained product is known as a compound. Compounds can be defined as substances consisting of 2 or more different types of elements in a fixed ratio of their atoms. When the elements combine, some individual property of the elements is lost and the newly formed compound has new properties.
Chemical Formula: Compounds are represented by their chemical formula. A chemical formula is a symbolic representation of the proportions of atoms that constitute a particular chemical compound.
The chemical formula of water is H2O which shows two atoms of hydrogen and one atom of oxygen have combined to form one molecule of H2O. The chemical formula for common salt is NaCl which shows one atom of sodium and one atom of chlorine combine to form one molecule of NaCl.
1. Types Of Compounds
Compounds can be classified into two types, molecular compounds and salts. In molecular compounds, the atom binds each other through covalent bonds. In salts, it is held together with ionic bonds. These are the two types of bonds out of which every compound is made of.
2. Example Of Compounds
- Example of compounds includes water (H2O), Hydrogen Peroxide (H2O2), etc. You could see water’s chemical formula, it says it has 2 atoms of Hydrogen combined with 1 atom of oxygen and in hydrogen peroxide, it has 2 atoms of hydrogen and two atoms of oxygen.
- Similarly, an example of salt would be the table salt (NaCl) which has 1 atom of sodium and one atom of chlorine.
Examples of some commonly used compounds and their molecular formula:
| Compound Name | Compound Formula |
| Alcohol | C2H6O |
| Acetic Acid | C2H4O2 |
| Sulphuric Acid | H2SO4 |
| Ammonia | NH3 |
| Methane | CH4 |
| Nitrous oxide | N2O |
| Salt | NaCl |
Recommended Videos
Chemical Bonding
What are the Elements?
We can define elements as a species of atoms that have the same number of protons in their atomic nuclei. Although an element’s atoms have the same number of protons, they can have different numbers of neutrons and hence different masses.
Isotopes: When atoms of the same element have different numbers of neutrons, they are known as isotopes. As of now, there are 118 elements, of which the first 94 are naturally occurring while the remaining 24 are synthetic elements.
Elements are complete chemical substances which relate to a single entry in the modern periodic table. Elements consist of one kind of atom only. They cannot be broken down into simpler fragments and can exist as atoms or as molecules. Elements are represented by symbols that are assigned by IUPAC. For example, Oxygen is represented by O, Aluminium is represented by Al, etc.
1. Types of Elements
The elements are arranged in the periodic table and are split depending upon their groups as either metallic or non-metallic. Metallic is further classified into Main Group Metals, Transition Metals, and f-block metals. These are again further divided, depending upon their properties.
2. Examples of Elements
Elements exist in their simplest form and cannot be broken down further. So, elements can exist in the form of ions, atoms, isotopes, molecules.
- An example of an element is Nitrogen atom(N), Nitrogen gas (N2), Nitrogen ion(N3–) and Nitrogen isotopes (Nitrogen-13, Nitrogen-14, and Nitrogen-15).
Similarly, you could see other elements’ existence.
Examples of some commonly used elements along with their chemical symbols:
| Name of the element | Chemical symbol |
| Hydrogen | (H) |
| Boron | (B) |
| Carbon | (C) |
| Silicon | (Si) |
| Sodium | (Na) |
| Lead | (Pb) |
| Platinum | (Pt) |
Frequently Asked Questions – FAQs
What does compound mean?
A compound is a material formed by chemically bonding two or more chemical elements. The type of bond keeping elements in a compound together may vary: covalent bonds and ionic bonds are two common types. The elements are always present in fixed ratios in any compound.
What is an example of a compound?
A compound is a material composed of two or more components. Water, carbon dioxide and table salt are some examples of compounds.
What is the classification of compounds?
There are two basic groups of compounds. We are characterized by the way the atoms in the compound bind to each other. Such two types are called “molecular” and “salt” compounds.
What is the meaning of the term element?
A material that can not be separated by chemical means into simpler substances. Each element consists of atoms with the same atomic number, that is, each atom has in its nucleus the same number of protons as all other atoms of that element.
Is pure substance an element?
A component is a single material that can not be divided into various types of substances. Carbon, oxygen, hydrogen, gold, silver and iron are examples of elements. Every element consists of just one atom form.
What is the difference between an element a compound and a mixture?
A material that only consists of one form of atom. Compound: A material which consists of more than one form of bonded atom. Mixture: a blend of two or more unbounded components or compounds; each component of the mixture preserves its own properties.
What are the two classifications of mixtures?
Two major groups can be divided into mixtures: homogeneous and heterogeneous. A homogeneous mixture is one in which the entire composition of its elements is combined evenly.
What is an atom element compound and mixture?
The same number of protons and electrons would be found in a single atom and most atoms contain at least as many neutrons as protons. An element is a material completely formed from one form of atom. A composite is a material consisting of chemically connected two or more separate elements.
Stay tuned with BYJU’S to learn interesting topics with engaging video lectures. For any further queries on this concept, please get in touch with the mentor support team at BYJU’S.

this is very helping. Thank you
It is very good to learn at home. It also helps me understand many tough concepts very well. Very easily I learn many things.
India’s no. 1 teachers help study better and also helping me combining my future.
Thank you.
really helpful am very grateful
This is a great app. Thanks.
this really helps
Thanks for this,very helpful
This is a grate app thanks for byju’s