What is Aluminium Sulphate (Al2(SO4)3)?
Al2(SO4)3 is a chemical compound with the chemical name Aluminium sulphate.
Aluminium sulphate is also called Filter Alum or Dialuminum trisulphate. It is a white crystalline solid in its anhydrous form and in its solution form it appears as a colourless liquid. Both the forms are non-toxic and non-combustible.
Aluminium sulphate is soluble in water but insoluble in ethanol. It is odourless and has a mildly astringent taste, sweet taste. On decomposing, it emits toxic fumes of sulphur oxides. The solution of aluminium sulphate is corrosive to aluminium. This compound is produced in the laboratory by adding aluminium hydroxide, to sulphuric acid.
Table of Contents
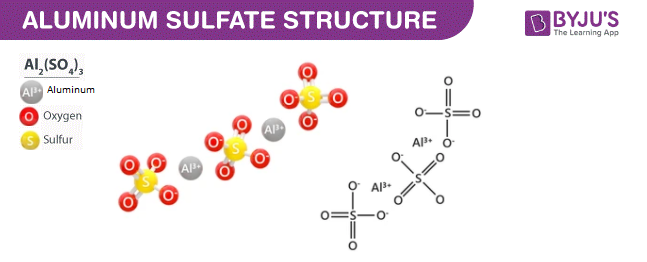
- Aluminium Sulphate Structure
- Properties of Aluminium sulphate
- Preparation of Aluminium Sulphate
- Uses of Aluminium sulphate
- FAQs
Aluminium Sulphate Structure (Al2(SO4)3 Structure)
Properties of Aluminium Sulphate – Al2(SO4)3
| Al2(SO4)3 | Aluminium sulphate |
| Molecular Weight/ Molar Mass | 342.15 g/mol |
| Density | 2.672 g/cm3 |
| Boiling Point | 214° F |
| Melting Point | 770 °C |
Preparation of Aluminium Sulphate
- Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to crystallize. Accessible as pure, lustrous crystals, granules, or powder.
2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6 H2O
- Aluminium sulphate is also produced by heating aluminum metal in a sulfuric acid solution.
2 Al + 3 H2SO4 → Al2(SO4)3 + 3 H2↑
Aluminium Sulphate (Al2(SO4)3 ) Uses
- It is used in baking soda.
- It is used for gardening to balance the soil PH.
- Used in the purification of water.
- It is used in the dyeing of cloth.
- It is used in making paper.
- Used for the printing on cloth.
- It is used in concrete as an accelerator and waterproofing agent.
- It is used in firefighting foam.
- It is used in sewage treatment.
- It is used as the fireproofing agent.
Frequently Asked Questions – FAQs
How is aluminium sulphate made?
Aluminium sulphate is formed by reacting with the correct amount of sulphuric acid to freshly precipitated aluminium hydroxide. The solution that results is then evaporated and allowed to crystallize. Accessible as pure, lustrous crystals, granules, or powder.
What type of compound is aluminium sulphate?
Aluminium sulphate is an ionic compound, a combination of both positive and negative ions. Ions are charged atom, which may either be monatomic ions (single atoms) or polyatomic (multiple atoms combined to form a charged part). Aluminium forms a + 3 ion, Al+3, and sulphate is the -2 polyatomic ion, (SO4)-2.
How dangerous is aluminium sulphate?
Aluminium sulphate is an irritant to the skin and eyes, so you should wear gloves and eye protection while dealing with it. It is mildly dangerous if aluminium sulphate is swallowed in any way because when the salt is swallowed it can form extremely corrosive sulphuric acid.
Why is aluminium sulphate soluble in water?
Aluminium sulphate is a chemical compound produced with Al2(SO4)3. It is soluble in water and is primarily used in purification of drinking water and wastewater treatment plants as a coagulating agent (promoting particle collision by neutralizing charge) as well as in paper processing.
What is the smell of Aluminium sulphate?
Aluminium sulphate is an odourless, white crystalline hygroscopic compound, moderately water-soluble and insoluble in organic solvents. This has an acidic flavour.
Learn more about the Structure, physical and chemical properties of Aluminium sulphate (Al2(SO4)3) from the experts at BYJU’S.
Other related links:
| Aluminium | Sulphur |


Comments