What is Hydroquinone?
Hydroquinone is an aromatic organic compound with a chemical formula C6H6O2.
Hydroquinone has two hydroxyl groups binding to a benzene ring in the para position. It is a melanin synthesis Inhibitor. It is also known as benzene-1, 4-diol or Quinol. It is a derivative of phenol and has antioxidant properties. It appears as a granular solid white in colour. The name Hydroquinone was coined in the year 1843 by Friedrich Wohler.
The most widely used industrial methods of producing Hydroquinone are hydroxylation of phenol and cumene process. Other less common methods are oxidation of various phenols, oxidation of aniline by manganese dioxide, and the dry distillation of quinic acid or from acetylene and iron pentacarbonyl. It is widely used for benzene exposure as a biomarker. It naturally occurs in the defensive glands of bombardier beetles.
Properties of Hydroquinone – C6H6O2
| C6H6O2 | Hydroquinone |
| Molecular Weight/ Molar Mass | 110.11 g/mol |
| Density | 1.3 g cm−3 |
| Boiling Point | 287 °C |
| Melting Point | 172 °C |
Hydroquinone Structure – C6H6O2
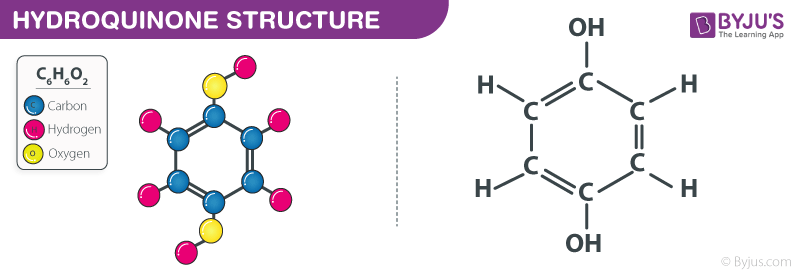
Hydroquinone Structure – C6H6O2
C6H6O2 Uses (Hydroquinone)
- It is used as a reducing agent
- It is used to treat melasma
- It is used in the prevention of methyl methacrylate
- It is used in skin whitening
- It is used for benzene exposure as a biomarker
- It is used by photographic developers
- It is used in the treatment of acne scars
- It is used in various cosmetic products
Frequently Asked Questions
How is aluminium sulfate made?
10 g of finely powdered quinone is suspended in cold water and saturated liquid with sulfur dioxide until full solution and decolouration have occurred after the intermediate formation of hydroquinone.
Is oxidized hydroquinone still effective?
No, the hydroquinone won’t harm you if it turns brown but if it has oxidized or weakened it won’t be successful either. I would consider tossing it out after a year or earlier if the tube turns dark.
What is an alternative to hydroquinone?
Mequinol (4-Hydroxyanisole) is the principal alternative to hydroquinone for a prescription. This is also known as methoxy phenol, monomethyl ether with hydroquinone, and p-hydroxyanisole. It was found to be just as effective as hydroquinone.
How long can you use hydroquinone safely?
Prescribe concentrations of hydroquinone not more than 4 per cent. Allow patients to take adequate sun protection using hydroquinone. Continue to administer hydroquinone for four to five months at the latest. Enable the skin to relax, and recover after hydroquinone therapy for two to three months.
Can you use hydroquinone long-term?
Of greater concern are persistent adverse effects associated with hydroquinone use. Such complications include ochronosis, discolouration of the fingers, conjunctival melanosis and degeneration of the cornea. Ochronosis is the most prevalent clinical condition associated with long-term hydroquinone use.
Also Read:
| Hydrogen Peroxide | Benzoyl Peroxide |
| Potassium Chloride | Hydroquinone |
Learn more about the methods of producing hydroquinone, its structure, and uses of Hydroquinone (C6H6O2) from the expert faculties at BYJU’S – India’s largest ed-tech company.

Comments