What is Ethane?
Ethane is a saturated hydrocarbon found in gaseous state. Ethane is the second simplest alkane followed by methane. It contains 2 carbon atoms and 6 hydrogen atoms. So the formula for ethane is C2H6. It is prepared by laboratory method using sodium propionate. Ethane is the most important gaseous fuel. Natural gas components of ethane and heavier hydrocarbons are quite easily separated from the gas stream and liquefied under moderate pressure.
Other names – Methyl methane, Bimethyl, Dimethyl, Ethyl hydride
| C2H6 | Ethane |
| Density | 1.36 kg/m³ |
| Molecular Weight/ Molar Mass | 30.07 g/mol |
| Boiling Point | -89 °C |
| Melting Point | -182.8 °C |
| Chemical Formula | C2H6 |
Synthesis of Ethane – C2H6
-
- Ethane is synthesized by reduction of ethyl iodide using zinc + copper couple in alcohol. The chemical equation is given below.
CH3CH2I + 2[H] → C2H6 + HI
-
- Ethane is also prepared by Wurtz reaction. When methyl bromide or methyl iodide and sodium are heated in the presence of dry ether ethane is formed.
CH3I + 2Na + CH3I → CH3– CH3 + 2NaI
Ethane Structure – C2H6
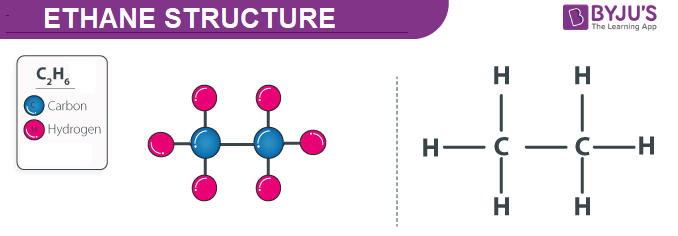
Physical Properties of Ethane – C2H6
| Odour | Odourless |
| Appearance | Colourless gas |
| Covalently-Bonded Unit | 7 |
| Heavy Atom Count | 2 |
| Nature of compound | Saturated compound |
| Solubility | Soluble in water |
Uses of Ethane – C2H6
- Used in the petrochemical industry as a fraction of that produced in the natural gas liquids plants alone.
- Used in the preparation of ethanol, acetaldehyde and acetic acid which find use in paints, varnishes, adhesive, plastic etc.
- Used as the most specific volatile marker for the investigation of lipid peroxidation.
- Used to make ethylene, for everything from antifreeze to plastics to ripening fruit.
Frequently Asked Questions
What is ethane used for?
Ethane has quite a range of applications. It is the second most abundant natural gas component, widely used in many homes. It is also used for the production of a chemical called ethylene, which is used in the manufacture of goods such as plastic, automotive antifreeze, and detergent.
Can ethane be classified as a hydrocarbon?
Ethane is a colourless, odourless, gaseous hydrocarbon (compound containing only hydrogen and carbon) which belongs to the paraffin subcategory. Its chemical formula is C2H6. Ethane is the simplest hydrocarbon since it contains only one carbon–carbon bond in its structure.
How is ethane produced?
For the removal of various impurities, oil and natural gas must be processed at first production. The processing of natural gas removes hydrocarbons such as ethane, butane, propane and other hydrocarbons from the gas stream, as well as water and other impurities.

Comments