What is Polyurethane Foam?
“Polyurethane foam is a linear polymer composed of organic units joined by links of carbamate. The chemical formula is C27H36N2O10“.
Polyurethane is another synthetic resin-type varnish. Polyurethane foam is formulated in different ways for different uses. Urethane is a carbonyl-containing functional group in which the carbonyl carbon is bonded to both an -OR group and an -NR2 group. Polyurethane is formed by reacting a hydroxyl-terminated polyether or polyester with an isocyanate.
Other names – Ethylene glycol copolymer
| C27H36N2O10 | Polyurethane Foam |
| Density | approximately 3 to 50 lbs |
| Molecular Weight/ Molar Mass | 548.589 g/mol |
| Boiling Point | 210 K |
| Melting Point | 330 K |
| Chemical Formula | C27H36N2O10 |
Table of Contents
- Polyurethane Foam Structure – C27H36N2O10
- Physical Properties of Polyurethane Foam –C27H36N2O10
- Chemical Properties of Polyurethane Foam – C27H36N2O10
- Uses of Polyurethane Foam – C27H36N2O10
- Frequently Asked Questions
Polyurethane Foam Structure – C27H36N2O10
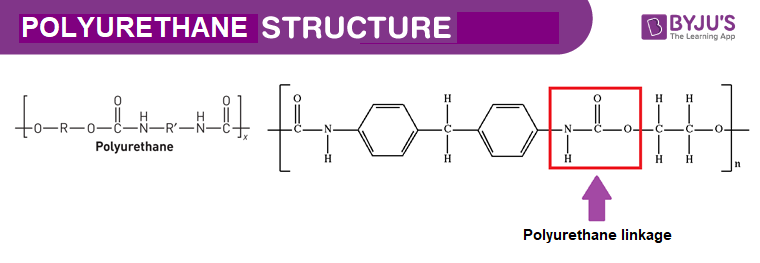
Physical Properties of Polyurethane Foam – C27H36N2O10
| Odour | No odour |
| Appearance | Solid with open cellular structure |
| Covalently-Bonded Unit | 2 |
| Hydrogen Bond Acceptor | 6 |
| Complexity | 338 |
| Solubility | Insoluble in water |
Chemical Properties of Polyurethane Foam – C27H36N2O10
- Polyurethanes undergoe oxidation forms Hydrazoic acid, water and carbon dioxide. The chemical equation is given below.
6C27H36N2O10 + 185O2 → 162CO2 + 106H2O + 4HN3
Uses of Polyurethane Foam – C27H36N2O10
- Polyurethanes can be used to make a multitude of items, from very simple, noncritical parts to products that are used in vital engineering applications.
- Used extensively where stretch and strength are required in fabrics.
- Used in technical fibre ropes, fishing lines, and some agricultural products.
- Used in cars and household furnishings, the polymer is used in the construction industry and in the manufacture of footwear and for coating and adhesives as well as textiles.
Frequently Asked Questions
What are the uses of polyurethane foam?
The uses of polyurethane foam are given as:
1. Polyurethane foam is a vital component of bathroom sponges and kitchen sponges and is, therefore, widely used in their manufacturing.
2. Upholstered furniture cushions are often manufactured with the help of flexible polyurethane foam.
3. The cores and the paddings of certain solid-core mattresses also employ flexible polyurethane foam as a component. Since this polymer is recyclable, these products are considered by many to be moderately environment-friendly.
4. It can be noted that some water tanks maintain the water temperature over long periods of time due to the presence of an insulating layer of polyurethane.
How is polyurethane produced?
Polyurethane is usually produced via the mixing of two separate liquid streams. In some cases, more than two liquid streams are mixed. The first stream, known as the polyol stream, usually contains surfactants and catalysts. This stream is also known to contain blowing agents. The other streams contain other important additives that determine the type of polyurethane that is produced as the final product. Types of substances that are used in the resin blend additive stream include cross linking agents, chain extending agents, pigments, fillers, and flame retardants.
Is polyurethane toxic?
Polyurethane is mostly chemically inert and is, therefore, not toxic under normal circumstances. However, this compound is classified as a combustible substance and must be kept away from open flames (since it may spontaneously ignite). Moreover, the decomposition reaction undergone by polyurethane can result in the formation of carbon monoxide gas, which is highly toxic to human beings. The combustion of this compound is also known to produce large quantities of hydrogen cyanide, another highly toxic substance. This is one of the reasons why polyurethane foams are usually treated with flame retardant substances during the production process.

Comments