What is Oxalic acid?
Oxalic acid is a dicarboxylic acid with a chemical formula C2H2O4. It is also known as Ethanedioic acid or Oxiric acid.
This organic compound is found in many vegetables and plants. It is the simplest dicarboxylic acid with condensed formula HOOC-COOH and has an acidic strength greater than acetic acid. Excess consumption of oxalic acid can be dangerous. It is produced by the oxidation of carbohydrates. It can also be prepared in the laboratory by the oxidation of sucrose in the presence of nitric acid and a catalyst like vanadium pentoxide.
Oxalic acid has a structure with two polymorphs and it appears as a white crystalline solid which becomes a colourless solution when dissolved in water. It is a reducing agent and is used as a chelating agent with oxalate as its conjugate base.
Table of Contents
Oxalic acid Formula
Oxalic acid is a dicarboxylic acid with the chemical formula C2H2O4. Oxalic acid occurs in the cell sap of Oxalis and Rumex species of plants as potassium and calcium salt.
In an aqueous solution, oxalic acid is a weak acid that will only partially ionise. Oxalic acid has two acidic protons. The initial ionisation yields HC2O4-, a weak acid that will ionise as well.
Oxalic acid is one of the most powerful of the organic acids and expels carbonic acid and many other acids from their salts. Oxalic acid is produced by the action of either hydrate of potash or of nitric acid upon most organic compounds of natural occurrence. It is also called diprotic acid.
Equivalent Weight of Oxalic Acid (Calculation)
The molar mass of hydrated oxalic acid is 126 grams per mole. Since the chemical formula of this compound can be written as COOH-COOH, it can be understood that oxalic acid is a dibasic acid which has the ability to donate two H+ ions. Therefore, the equivalent weight of oxalic acid can be calculated with the help of the following formula:
Equivalent weight = (molecular weight)/(number of equivalent moles)
Since 1 mole of oxalic acid can release 2 moles of H+ ions and neutralize 2 moles of OH– ions, the number of equivalent moles here is equal to 2. Thus, the equivalent weight of oxalic acid can be calculated as follows:
Equivalent mass of oxalic acid = molecular mass of oxalic acid/2 = 126g/2 = 63 grams.
Therefore, the equivalent weight of oxalic acid is 63 grams.
Oxalic acid Structure – C2H2O4
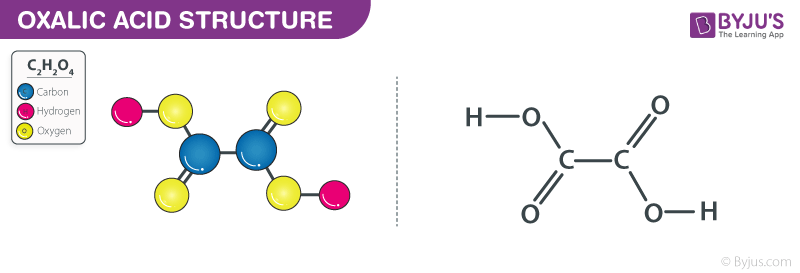
Oxalic acid Structure
In its anhydrous form, it can be noted that oxalic acid exists in two different polymorphs. In the first polymorph of oxalic acid, hydrogen bonding takes place. Due to this hydrogen bonding, a chain-like structure is developed at the intermolecular level. The second polymorph of this compound is also subject to hydrogen bonding. However, in this case, the hydrogen bonding attributes a sheet-like structure to the compound at the intermolecular level. This compound is widely used in esterification reactions owing to two important properties. The first property that makes oxalic acid ideal for esterification reactions is its acidic nature. The second and most important property of oxalic acid is its hydrophilic nature (it tends to seek water).
Preparation of Oxalic acid – C2H2O4
Oxalic acid can be easily prepared by oxidation of certain carbohydrates like sucrose by concentrating nitric acid. During oxidation, the carbon atoms are split off in pairs giving oxalic acid.
Procedure
-
-
-
- Take 10gm of cane sugar in a 250ml conical flask.
- Add 50ml of concentrated nitric acid and heat on a boiling water bath.
- The mixture becomes warm and most of the sugar gets dissolved.
- Remove the flask and keep on the wooden surface. After 15min pour the hot solution in an evaporating dish, washing the flask with 10ml of concentrated nitric acid and adding to the evaporating dish.
- Evaporate the acid solution by heating on the boiling water bath until the volume is reduced to about 10ml.
- Cool the solution in the ice water mixture.
- Oxalic acid crystallizes out rapidly.
- Collect the product on the Buchner funnel at the pump.
- Dry the product by pressing between the filter paper and then in the air.
- The yield of the product is about 3.5gm of oxalic acid.
-
-
Properties of Oxalic acid – C2H2O4
| C2H2O4 | Oxalic acid |
| Molecular Weight/ Molar Mass | 90.03 g/mol |
| Density | 1.90 g cm−3 |
| Appears | White crystals |
| Melting Point | 189 to 191 °C |
C2H2O4 Uses (Oxalic acid)
-
-
-
- It is used in the dyeing process as a mordant
- It is used in removing rust
- In lanthanide chemistry, it is used as an important reagent
- It is applied on marble sculptures to make it shine
- It is used in the manufacture of dye
- It is used in bleaches
- It is used in removing food and ink stains
- It is used in developing photographic film
- It is used in wastewater treatment to remove the calcium deposit.
-
-
Oxalic acid’s conjugate base is the hydrogen oxalate anion and its conjugate base (commonly known as oxalate) is a competitive lactate dehydrogenase (often abbreviated to LDH) enzyme inhibitor. LDH catalyses the conversion of pyruvate to lactic acid (end product of the fermentation, which is an anaerobic process) oxidizing coenzyme NADH to NAD+ and H+ at the same time. Restoring NAD+ levels is necessary if anaerobic energy metabolism is to continue through glycolysis. Because cancer cells preferentially use anaerobic metabolism, LDH inhibition has been shown to inhibit tumour development and growth. Thus, this compound provides an interesting possible course for the treatment of certain cancers.
Health Hazard
Oxalic acid is a strong poison. The toxic symptoms from ingestion include vomiting, diarrhoea, and severe gastrointestinal disorder, renal damage, shock, convulsions, and coma. Death may result from cardiovascular collapse. Oxalic acid is an irritant of the eyes, mucous membranes, and skin. Inhalation or ingestion may result in kidney damage.
Oxalic acid is a strong poison. The toxic symptoms include renal damage, shock, convulsions. The toxicity arises as oxalic acid reacts with the calcium in the tissues to form calcium oxalate, thereby upsetting the calcium potassium ratio. The deposition of oxalates in the kidneys tubules may result in kidney damage.
Oxalic acid poisoning can result in headaches, dizziness, nausea, vomiting, convulsions, coma, and even death. A skin rash, discomfort, redness, blisters, and slow-healing ulcers can result from prolonged or recurrent exposure.
Frequently Asked Questions
Is oxalic acid a strong acid?
As an organic acid, oxalic acid is a weak acid. Oxalic acid is known to be a soft acid. It’s weaker than (water) H3O+ atom. But it is better than acetic acid, benzoic acid, and so on.
What is the pH of oxalic acid?
The constant acid dissociation of oxalic acid is 5.4 × 10-2. As oxalic acid is a polyprotic acid, there are two values and it has a chemical formula HOOC-COOH.
What is the world’s strongest acid?
Carborane superacids can be considered the strongest polar acid in the world since fluoroantimonic acid is a combination of hydrofluoric acid and pentafluoride antimony. The pH value of Carborane is -18.
What is the basicity of oxalic acid?
Acid weight equivalent = (acid molecular weight)/Basicity. Oxalic acid basicity is = 2. Oxalic acid (H2C2O4) molecular weight = 90.
What is the Valency of oxalic acid?
The valence factor is the no. of acid and base produced H+ or OH-ion. The acid releases 2 H+ ion, hence the valence factor is 2 in the case of oxalic acid. While the no. of e exchanged by a molecule is called that compound’s valence factor for that reaction in the case of the redox reaction.
Is oxalic acid polar or nonpolar?
Two carboxylic acid groups are composed of oxalic acid (HOOC-COOH). These groups are polar and are able to form hydrogen bonds with molecules of water. Oxalic acid is therefore an ideal water-solute. In fact, it is polar and is thus soluble in all polar solvents.
What is the natural source of oxalic acid?
The most popular constituent of kidney stones is calcium oxalate. Early researchers isolated Wood-sorrel (Oxalis) oxalic acid. Members of the family of spinach and brassicas (cabbage, broccoli, brussels sprouts) are rich in oxalates, as are sorrels and umbellifers such as petersil.
How do you neutralize oxalic acid?
Since this is an acid it must be neutralized before any finish can occur. To neutralize the acid two to three times, flood the surface with clean water and baking soda and leave it to dry at least overnight.
Also, Read:
Preparation of Standard Solution of Oxalic Acid
| Benzoic Acid | Boric Acid |
| Sodium Hydroxide | Phosphoric Acid |
To learn more about oxalic acid and other related chemical compounds, register with BYJU’S and download our app.


Comments