What is Ethylene Glycol?
Ethylene Glycol is dihydroxy alcohol with a chemical formula C2H6O2. Ethylene glycol is also called ethane 1,2 diol.
This organic compound is highly toxic. It is also known as Ethane-1,2-diol or Monoethylene glycol. It has no smell and is viscous. It is colourless and has a sweet taste. It appears as a clear, colourless, liquid. It is widely used as an antifreeze and a raw material in the plastic industry. Ethylene Glycol is produced when ethylene oxide reacts with water.
Ethylene glycol has been the most abundantly produced diol because it is one of the monomers of polyethylene terephthalate. Ethylene glycol has been synthesized by the oxidation of ethylene with O2 to ethylene oxide and the subsequent hydration of ethylene oxide to ethylene glycol. Usually, ethylene is supplied from the thermal cracking of naptha from petroleum refining.
Preparation of Ethylene Glycol – C2H6O2
Ethylene glycol is a prominent member of the class of dihydroxy alcohols known as ethylene glycols, dihydric alcohols or diols. Ethylene glycol is prepared by the following methods.
1. From Ethylene
By hydroxylation of ethylene. When the ethylene is treated with Baeyer’s reagent (cold dilute alkaline solution of potassium permanganate), hydroxylation takes place at both the carbon atoms.

Preparation of Ethylene Glycol From Ethylene

2. From ethylene oxide
By hydrolysis of ethylene oxide. Ethylene oxide obtained after catalytic oxidation of ethylene is hydrolysed in the presence of dilute acid or base at high temperature to ethylene glycol.
Preparation of Ethylene Glycol From Ethylene Oxide.

3. From Oxalic esters
Oxalic esters on reduction with sodium and alcohol give ethylene glycol.

Properties of Ethylene Glycol – C2H6O2
| C2H6O2 | Ethylene Glycol |
| Molecular Weight/ Molar Mass | 62.07 g/mol |
| Density | 1.11 g/cm³ |
| Boiling Point | 197.3 °C |
| Melting Point | -12.9 °C |
Ethylene Glycol Structure – C2H6O2
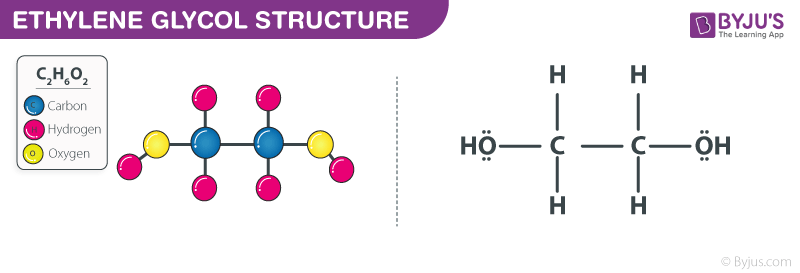
Ethylene Glycol Structure – C2H6O2
C2H6O2 Uses (Ethylene Glycol)
- It is used in the manufacture of polyester as a raw agent
- It is used in antifreeze formulation
- It is used in air conditioning systems
- It is used as a precursor in the plastic industry
- It is used in convective heat transfer
- It is used as a dehydrating agent in the gas industry
- It is used as a desiccant
- It is used as an additive for the prevention of
- It is used in the manufacturing of capacitor
- It is used to preserve biological specimens
- It is used as an ingredient in shoe polish
- It is used in the manufacturing of some vaccines
Recommended Videos
Identification of Alcohols – Primary, Secondary And Tertiary

Frequently Asked Questions
What is ethylene glycol used for?
Ethylene glycol is a chemical that is widely used in many commercial and industrial applications including antifreeze and refrigerants. Ethylene glycol helps avoid the freezing of your car’s engine in winter, and serves as a coolant to minimize overheating in summer.
Is ethylene glycol an alcohol?
Ethylene glycol, also known as ethane-1,2-diol, is the simplest member of the organic compound family glycol. A glycol is an alcohol on adjacent carbon atoms, with two hydroxyl groups. The common name ethylene glycol simply means “ethylene derived glycol.”
What is the common name of ethylene glycol?
Ethylene glycol (name IUPAC: ethane-1,2-diol) is an organic formulated compound (CH2OH)2. It is used mainly for two reasons, as a raw material in the manufacture of polyester fibers and formulations for antifreeze. This is an odourless, colourless, viscous liquid with a sweet taste.
Is ethylene glycol corrosive?
Ethylene glycol is a Corrosion Source. Most corrosion from ethylene glycol is caused by decomposition at higher temperatures of organic acids (such as glycolic acid).
Is ethylene glycol polar?
First, ethylene glycol comprises polar O-H groups; they are polar since oxygen is much more electronegative than hydrogen, and thus appears to polarize the pair of electrons in the O-H bond.
Also, Read:
| Urea | Carbohydrates |
| Glucose | Phenol |
To know more about the production, uses, and structure of Ethylene Glycol (C2H6O2) register now. Learn from the expert faculties at BYJU’S – India’s largest education company.

Comments