What is Phenol? (Carbolic Acid)?
Phenol is an aromatic compound. The chemical formula of this organic compound is C6H6O. Phenol is also known as Carbolic acid.
It consists of a hydroxyl group and a phenyl group attached to each other. It considerably dissolves in water. Earlier it was used as carbolic soap. It is mildly acidic and is corrosive to the respiratory tract, eyes, and skin.
Phenol is a crystalline solid white in colour and needs to be handled with care as it can cause chemical burns. Friedlieb Ferdinand Runge discovered Phenol in the year 1834. It was extracted from coal tar. It is also known as phenolic acid. If a compound is consisting of a six-membered aromatic ring and bonded to a hydroxyl group directly, then it can be referred to as phenol.
Table of Contents
- Structure of Phenol (Carbolic acid)- C6H6O
- Properties of Phenol (Carbolic acid)- C6H6O
- Natural Sources of Phenol (Carbolic acid)- C6H6O
- Nomenclature of Phenol (Carbolic acid)- C6H6O
- Synthesis of Phenol (Carbolic acid)- C6H6O
- Chemical Reactions of Phenol (Carbolic acid)- C6H6O
- Uses of Phenol (Carbolic acid)- C6H6O
- Frequently Asked Questions
Structure of Phenol (Carbolic acid)- C6H6O
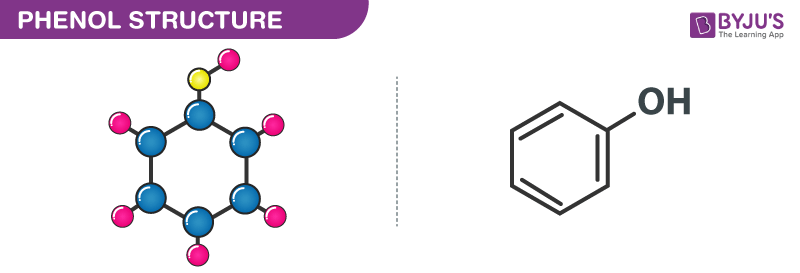
Phenol Structure – C6H5OH
Properties of Phenol (Carbolic acid)- C6H6O
| C6H6O | Phenol |
| Molecular Weight/ Molar Mass | 94.11 g/mol |
| Density | 1.07 g/cm³ |
| Melting Point | 40.5 °C |
| Boiling Point | 181.7 °C |
Natural Sources of Phenol (Carbolic acid)- C6H6O
- Phenol is a constituent of coal tar and is formed during the decomposition of organic materials. Increased environmental levels of phenol may result from forest fires. It has been detected among the volatile components from liquid manure.
- Industrial sources of phenols and other related aromatics from a petroleum refinery, petrochemicals, basic organic chemical manufacture, coal refining, pharmaceuticals, tannery and pulp, and paper mills.
Nomenclature of Phenol (Carbolic acid)- C6H6O
Phenols are organic compounds containing at least one -OH group directly attached to the benzene ring. Depending upon the number of hydroxyl groups attached to the benzene ring, phenols can be classified as monohydric, dihydric and trihydric phenols.
- Monohydric phenols – The simplest member of the series is hydroxybenzene, commonly known as phenol, while others are named substituted phenols. The three isomeric hydroxyl toluenes are known as cresols.
- Dihydric phenols – The three isomeric dihydroxy benzenes namely catechol, resorcinol, and quinol are better known by their common names.
- Trihydric phenols – Trihydroxy phenols are known by the common names called pyrogallol, hydroxyquinol and phloroglucinol.
Synthesis of Phenol (Carbolic acid)- C6H6O
Phenols can be synthesized by the following methods.
1. From sulphonic acids (by alkali fusion of sodium benzene sulphonate)
The first commercial process for the synthesis of phenol. Sodium benzene sulphonate is fused with sodium hydroxide at 573K to produce sodium phenoxide, which upon acidification yields phenol.

Synthesis of Phenols From Sulphonic Acids
2. From diazonium salts (by the hydrolysis of diazonium salt – laboratory method)
When a diazonium salt solution is steam distilled or is added to boiling dil.H2SO4, it forms phenol.

Synthesis of Phenols From Diazonium Salts
Chemical Reactions of Phenol (Carbolic acid)- C6H6O
A hydroxyl group is attached to an aromatic ring and it is strongly activating ortho/para director, phenols possess considerable reactivity at their ortho and para carbons toward electrophilic aromatic substitution.
1. Reactions of the Aromatic Ring
The -OH group in phenol is ortho and para directing because it increases electron density at ortho and para positions due to resonance. Thus phenol undergoes electrophilic substitution reactions.
2. Halogenation
Like -NH2 group, -OH group is so much activating that it is rather difficult to prevent poly substitution.
If it is required to arrest the reaction at the mono substitution stage, the reaction should be carried out in non-polar solvents like CCl4 and CS2 and at lower temperatures.
Uses of Phenol (Carbolic acid)- C6H6O
- It is used as a precursor in drugs
- It is used as an antiseptic
- It is used in the production of nylon
- It is used to preserve vaccines
- It is used in oral analgesics
- Derivatives of phenol are used in beauty products like hair colour and sunscreen
- It is used in the synthesis of plastics
- It is used to produce detergents and carbonates
Also Read:
| Glycerin | Hydrochloric Acid |
| Ascorbic Acid | Phenolphthalein |
Frequently Asked Questions
What is phenol used for?
Phenol is so cheap it attracts plenty of small-scale applications. This compound is a part of industrial paint strippers used for the removal of epoxy, polyurethane, and other chemically resistant coatings in the aviation industry. Phenol derivatives can be used in cosmetics preparation, including sunscreens, hair colouring, skin lightening preparations, and skin toners/exfoliators.
Is phenol acidic or basic?
Phenol can be considered a weak acid. It is in equilibrium with the phenolate anion C6H5O− (also called phenoxide) in aqueous solutions that are within the pH range 5-6. One reason, for why phenol is more acidic than aliphatic compounds, is that it contains an OH group and the aromatic ring resonance stabilizes the phenoxide anion.
Comment on the solubility of phenol in water
Phenol is an organic compound which is considerably soluble in water, dissolving about 84.2 g in 1000 mL (to form a 0.895 M solution). Homogenous phenol-water mixtures at phenol to water mass ratios of ~2.6 and higher are possible. The phenol sodium salt, sodium phenoxide, is much more soluble in water.
Which is known as carbolic acid?
Phenol is also known as carbolic acid. It is an aromatic organic compound with the molecular formula C6H5OH.
Learn more about the molecular weight, structure, and properties of C6H6O from the expert faculties at BYJU’S.


Comments