Table of Contents
- What is Potassium Oxide?
- Potassium Oxide Structure – K2O
- Physical Properties of Potassium Oxide – K2O
- Chemical Properties of Potassium Oxide – K2O
- Uses of Potassium Oxide – K2O
- Frequently Asked Questions
What is Potassium Oxide?
Potassium oxide is an ionic compound formed by combining potassium and oxygen. It carries the chemical formula K2O. Potassium cannot be found free because it is too reactive. It has valency +1 and combines readily with oxygen atoms forming K2O. The oxide, K2O, is obtained as a grey crystalline substance when potassium is oxidised; potassium is burnt in excess oxygen to form potassium oxide. Potassium oxide is a strongly corrosive alkali, when dissolved in water.
Other names – Potassium monoxide, dipotassium hydroxide, Kalium oxide
| K2O | Potassium Oxide |
| Density | 2.35 g/cm³ |
| Molecular Weight/ Molar Mass | 94.2 g/mol |
| Charge | 0 |
| Melting Point | 740 °C |
| Chemical Formula | K2O |
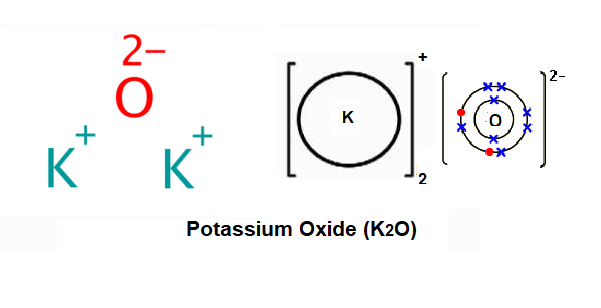
Potassium Oxide Structure – K2O
Physical Properties of Potassium Oxide – K2O
| Odour | Odourless |
| Appearance | Pale yellow solid |
| Heat capacity | 83.62 J/mol·K |
| Complexity | 2.8 |
| Solubility | Soluble in EtOH and ether |
Chemical Properties of Potassium Oxide – K2O
-
- Potassium oxide on treatment with water forms potassium hydroxide. The chemical equation is given below.
K2O + H2O → 2KOH
-
- Potassium oxide reacts with hydrogen chloride to form potassium chloride and water. The chemical equation is given below.
Uses of Potassium Oxide – K2O
- Potassium oxide or “pure potash” expressed as K2O, has been designated the commercial standard or unit.
- Used in farming as a fertiliser, but it can also be used in the manufacture of glass and soap, and in small quantities, it is useful for medical purposes.
- Used to treat fungal granulomatous disease and infections associated with zygomycetes.
- Used for over 100 years in the treatment of actinomycosis and actinobacillosis in cattle; it is also employed in the treatment of sporotrichosis and botryomycosis.
Frequently Asked Questions
What is potassium oxide used for?
It is widely used in the agricultural industry as a fertiliser. Potassium oxide is also used in the manufacturing of soaps and also in the manufacturing of glass. Certain medical processes are also known to involve potassium oxide.
Is potassium oxide acidic or basic?
Potassium oxide is a basic oxide. Other important examples of basic oxides include FeO (iron oxide) and CaO (calcium oxide).
How is potassium oxide produced?
Potassium oxide can be produced by reacting potassium with oxygen and treating the resulting potassium peroxide with metallic potassium. The chemical equation for this reaction is given by:
2K + K2O2 → 2K2O

Comments