Table of Contents
What is Phosphate (PO43-)?
PO43- is a chemical derivative of phosphoric acid with a chemical name Phosphate.
Phosphate is also called Phosphate ion or Orthophosphate. It is a trivalent inorganic anion and a conjugate base of hydrogen phosphate. One group of these compounds is composed of a group of salts containing the phosphate ion, the dihydrogen phosphate ion, or the hydrogen phosphate ion and positively charged ions such as calcium or sodium; another group is composed of esters. It is naturally found in food, human bodies, and water.
In the human body, it is present in bones, genes, and teeth. Phosphate is found in many phosphate minerals. Morocco is the largest global exporter and producer of phosphates.
Properties of Phosphate – PO43-
| PO43- | Phosphate |
| Molecular Weight/ Molar Mass | 94.97 g/mol |
| Hydrogen bond acceptor | 4 |
| Conjugate acid | Hydrogen phosphate |
| Heavy Atom Count | 5 |
Phosphate Structure (PO43- Structure)
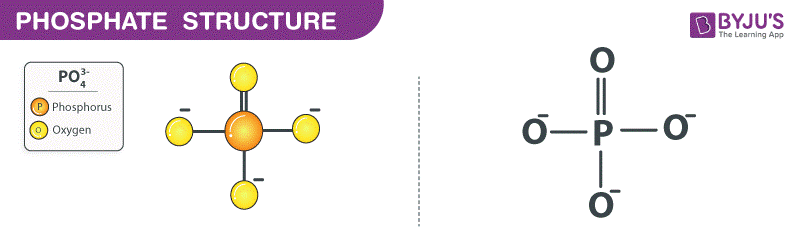
Phosphate Structure – PO43-
Phosphate (PO43- ) Uses
-
-
-
- It is used in toothpaste as a polishing agent.
- It is used in pharmaceuticals and personal products.
- Tricalcium phosphate is used in the toothpaste as a conditioning agent which helps the paste flow freely out of the tube.
- It is used in fire extinguishers.
- It is used in institutional and industrial cleaners.
-
-
Frequently Asked Questions
What is phosphate used for in plants?
Phosphorus is especially noted for its function in absorbing and transforming energy from the sun into useful compounds for plants. All DNA and RNA structures are connected by phosphorous bonds. Phosphorus is a vital component of ATP, the plant’s energy unit.
How is phosphorus used in everyday life?
Phosphorus is an essential nutrient in plants and its main use is in fertilizer production. Just as there are biological cycles of carbon and nitrogen, there is a phosphorus cycle. Phosphorus is used for the production of safety matches (red phosphorus), pyrotechnics, and fire shells.
Why do we need phosphate?
In order to keep your bones strong and safe, we need phosphorus to help make energy and push your muscles. Phosphorus also helps: develop healthy teeth and bones. Flush out kidney waste.
What is the importance of phosphorus?
Phosphorus’s principal role is in the creation of bones and teeth. It plays a significant role in the way carbohydrates and fats are used in the body. Protein for the growth, maintenance and repair of cells and tissues is also needed for the body to produce.
What are the sources of phosphorus?
High levels of phosphorus are present in protein foods such as milk and milk products and meat and alternatives such as beans, lentils and nuts. Grains, in particular whole grains, give phosphorus. Phosphorus is present in vegetables and fruit in smaller quantities.
Other related links:
| Hydrogen | Phosphorus |


Comments