What are Electrons?
Electrons are subatomic particles that hold an elementary charge of magnitude -1. The charge of an electron is equal in magnitude to the charge held by a proton (but has an opposite sign).
Therefore, electrically neutral atoms/molecules must have an equal number of electrons and protons. Although the magnitude of the charges held by protons and electrons are the same, the size and mass of an electron are much smaller than that of a proton (the mass of an electron is roughly 1/1837 the mass of a proton).
Table of Contents
- Charge of Electrons
- Mass of Electrons
- Who Discovered the Electron?
- Charge of Electrons
- Mass of Electrons
- Recommended Videos
- Electrons and Compounds
- Frequently Asked Questions – FAQs
Charge of Electrons
An electron is a negatively charged particle. The negative charge is equal to 1.602 × 10-19 coulomb in magnitude. The mass of an electron is 1/1837 of a proton.
Mass of Electrons
The mass of an electron is 9.10938356 × 10-31 kilograms. The mass of the electron is negligible compared to the mass of the proton.
Owing to their small size and mass, the properties exhibited by electrons can be studied better with the help of quantum mechanics rather than classical mechanics. This is because matter behaves differently at the quantum scale. For example, the uncertainty associated with the position and the velocity of an electron is much greater than that associated with a proton or a neutron, as per Heisenberg’s uncertainty principle. Electrons are distributed around the nuclei of atoms in atomic orbitals, which can be simply visualized as regions around the nucleus in which the probability of finding a specific electron is the highest.
Who Discovered the Electron?
The electron was discovered by the English physicist J.J.Thomson in the year 1897, via his experiments with cathode ray tubes.

Electrons – The Sub atomic Particles
Charge of Electrons
An electron is a negatively charged particle. The negative charge is equal to 1.602 × 10-19 coulomb in magnitude. The mass of an electron is 1/1837 of a proton.
Mass of Electrons
The mass of an electron is 9.10938356 × 10-31 kilograms. The mass of the electron is negligible compared to the mass of the proton.
Recommended Videos

Electrons and Compounds
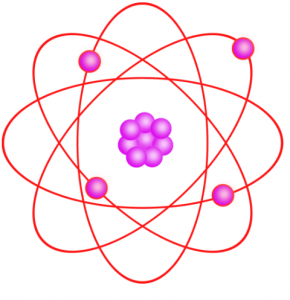
Later, scientists discovered the fundamental sub-atomic particles protons, electrons, and neutrons. An atom has a bulk mass at its centre called a nucleus. The nucleus contains the protons and the neutrons. Considering the solar system, it has been observed that the sun is at its center, and the planets revolve around it. Similarly, in an atom, the nucleus is at the center, and the electrons revolve around the nucleus.
The above image clearly shows the structure of an atom and its similarity with the structure of the solar system. The planets in the case of solar system or electrons in the event if an atom revolves around the sun and the nucleus respectively.
The credit for the discovery of electron goes to J.J. Thompson. He performed an experiment on the cathode rays; he showed that the cathode rays are charged particles. The velocity of cathode rays was much less than compared to the speed of light. He devised methods to measure the charge to mass ratio of cathode rays. Thompson, therefore, concluded that the cathode rays are 1/1000th mass of hydrogen ion (which is a proton). These negatively charged cathode ray particles are now known as electrons.
Electrons are negatively charged particles with negligible mass. The mass of an atom is the sum of the number of protons and neutrons. Protons are positively charged particles whereas neutrons do not carry any charge. The number of protons and electrons is equal hence an atom is electrically neutral in nature. Protons and neutrons together are known as nucleons.
Frequently Asked Questions – FAQs
Are electrons positive or negative?
Because a proton has a positive charge (+) and an electron has a negative charge (-), element atoms are neutral, with all positive charges cancelling out all negative charges. The number of protons, neutrons, and electrons in an atom varies from one to the next.
Who named Electron?
G. Johnstone Stoney invented the term “electron” in 1891 to describe the unit of charge discovered in tests that conveyed electric current through chemicals. J.J. Thomson’s Cambridge classmate Joseph Larmor used the phrase in this context.
Do protons and electrons have the same mass?
Electrons are a sort of negative-charged subatomic particle. Protons and neutrons have about the same mass as electrons, yet they are both significantly more massive (approximately 2,000 times as massive as an electron). A proton’s positive charge is the same magnitude as an electron’s negative charge.
Are protons and electrons equal?
Protons and electrons are in equal proportions in an atom. Because protons and electrons have equal and opposing charges, atoms are generally neutral.
Why do electrons repel each other?
A free electron will flow in the opposite direction of the force lines since it has the opposite charge qualities as a positive charge. As a result, an electron will change from a negative charge to a positive charge.
This article contains the basic properties of electrons. For any further queries or more content on this topic register to BYJU’S.


wow byjus is so good i think it is the best online education platform in world thank byjus for such a helpful app and website to study