What is Phenyl?
Phenyl group is formed when a hydrogen atom is removed from the benzene ring. It is represented using the symbol Ph in compounds sometimes.*
Any functional group containing an aromatic group, an aryl group is represented by Ar.
Other names – Phenyl radical
| C6H5 | Phenyl |
| Density | 1.248 g/mL at 25 °C |
| Molecular Weight/ Molar Mass | 77.106 g/mol |
| Boiling Point | 232.2±7.0 °C at 760 mmHg |
| Melting Point | −30 °C |
| Chemical Formula | C6H5 |
Table of Contents
- Structure of Phenyl – C6H5
- Physical Properties of Phenyl – C6H5
- Chemical Properties of Phenyl – C6H5
- Uses of Phenyl – C6H5
- Frequently Asked Questions
Structure of Phenyl – C6H5
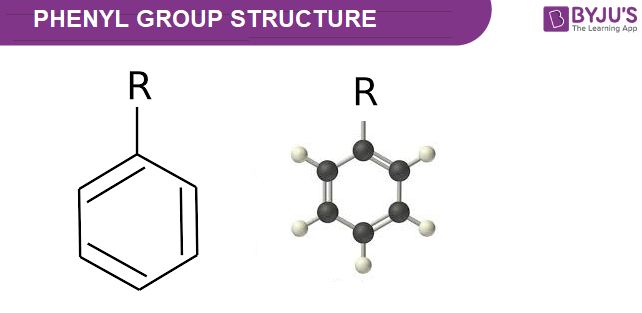
Physical Properties of Phenyl – C6H5
| Odour | Phenyl smell |
| Appearance | White crystalline solid |
| Covalently-Bonded Unit | 1 |
| Heavy Atom Count | 6 |
| Complexity | 27 |
| Solubility | Poorly soluble in water |
Chemical Properties of Phenyl – C6H5
-
- Phenyl group as phenol reacts with bromine solution forms bromo substituted phenol and hydrogen bromide. The chemical equation is given below.
2C6H5OH + 6Br2 → 2C6H2Br2OH + 6HBr
Uses of Phenyl – C6H5
- Used today in combination with other phenolics in various institutional and domestic disinfectant formulations.
- Used to destroy odour and promote sanitation.
- Used in public places like schools, hotels, stores and offices as a disinfectant.
- Possesses pharmacological properties and is used as antioxidant, analgesics, choleretic, etc.
Frequently Asked Questions
What are phenyl groups made up of?
A cyclic group of atoms with the formula C6H5 is a phenyl group or phenyl ring. Phenyl groups are closely related to benzene and can be described as a benzene ring, minus a hydrogen, which can be substituted as a functional group by any other element or compound. Phenyl groups have six carbon atoms in a hexagonal planar structure, of which five are bonded to hydrogen atoms.
Comment on the occurrence of phenyl groups in organic chemistry.
Phenyl groups are present in many natural as well as synthetic organic compounds. Amino acid phenylalanine is the most popular among natural products, and it contains a group of phenyls. A big petrochemical industry product is the “BTX” consisting of benzene, toluene, and xylene, which are all building blocks for phenyl compounds.
Do phenyl groups readily undergo oxidation or reduction?
Phenyl groups tend to resist reduction as well as oxidation. Phenyl groups (similar to all other aromatic compounds) have relatively higher stability than aliphatic (non-aromatic) groups. This enhanced stability is due to the special properties of molecular orbitals in an aromatic form.


Comments