What is Toluene?
Toluene is a clear, colourless liquid with a benzene-like odour. The chemical formula of toluene can be written as C6H5CH3.
Toluene is a naturally occurring compound derived primarily from petroleum or petrochemical processes. Toluene is a common component in gasoline, glues, and paint products. Toluene is a liquid, which is colourless, water-insoluble and smells like paint thinners. It is a mono-substituted colourless liquid, consisting of a CH3 group that is attached to a phenyl group.
Table of Contents
- Properties of Toluene
- Toluene structure
- Toluene Production
- Toluene Uses
- Toluene as a Precursor to Other Chemicals
- Toluene as a Solvent
- Other Applications of Toluene
- FAQs
Properties of Toluene
Toluene is more reactive to electrophiles than benzene. Due to the greater part of the methyl group than the electron-releasing properties, it reacts normal fragrant in the same position. It faces sulphonation to provide an acid called p-toluenesulfonic and chlorination by Cl2 in the presence of FeCl3 to give ortho and para isomers of chlorotoluene.
| Chemical formula of Toluene | C6H5CH3 |
| Boiling point of Toluene | 111 °C |
| Melting point of Toluene | −95 °C |
| Density of Toluene | 0.87 g/mL |
| Molecular weight of Toluene | 92.141 g/mol |
Toluene structure
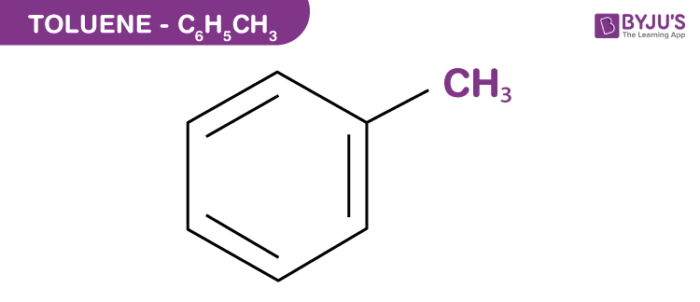
Toluene is widely used as an industrial raw material and a solvent for manufacturing many commercial products, including paints and glues.
Toluene Production
Toluene is naturally found in crude oil and as a by product in gasoline production. Also, it is obtained as a by product in coke production from coal.
Production of Toluene at the industrial level is inexpensive. It is synthesized in various methods. For example, the reaction of benzene with methyl chloride in the presence of aluminium chloride (Lewis acid) to give toluene:
- C6H5H + CH3Cl → C6H5CH3 + HCl
Toluene Uses
Toluene is widely used as a precursor to benzene. The chemical equation for the reaction between toluene and hydrogen gas can be written as follows.
C6H5CH3 + H2 → C6H6 + CH4
While the second most used application involves a disproportionate to a mixture of benzene and xylene.
Toluene as a Precursor to Other Chemicals
Along with the synthesis of benzene and xylene, toluene is used in the manufacture of the following
- Polyurethane foam
- Trinitrotoluene – Explosive
- TNT
- Synthetic Drugs.
Toluene as a Solvent
Toluene is a common solvent used for the following:
- Glues
- Paints
- Paint Thinners
- Printing Ink
- Rubber
- Leather Tanners
- Silicone Sealants
- Chemical Reactants
- Lacquers
- Disinfectants.
Other Applications of Toluene
It can be used in internal combustion engines as gasoline fuel.
Niche Applications of Toluene
It is used as a solvent for carbon nanomaterials, nanotubes and fullerenes.
Frequently Asked Questions – FAQs
What is toluene used for?
Is toluene soluble in water?
What does toluene smell like?
What functional group is toluene?
How many sigma bonds are in toluene?
To learn more about toluene and its properties, register with BYJU’S Now!


Comments