What is Sodium chloride?
Sodium chloride is an ionic compound with the chemical formula NaCl.
Sodium chloride is also known as salt. It occurs in oceans and sea waters. It is also found as rock salt. About 1% to 5 % of seawater is made of NaCl. It is a crystalline solid, white. In its aqueous form, it is called a saline solution.
The molecular weight of NaCl is 58.44g/mol
This compound is water-soluble and consists of sodium cation and chloride anion. The sodium and chloride ions are present in the ratio of 1:1. It is widely known as table salt and is mostly used in the food industry for preservation and flavouring. The pH of sodium chloride is 7.
Properties of Sodium chloride – NaCl
| NaCl | Sodium chloride |
| Molecular Weight/ Molar Mass of sodium chloride | 58.44 g/mol |
| Density of sodium chloride | 2.165 g/cm3 |
| Boiling Point of sodium chloride | 1.413 °C |
| Melting Point of sodium chloride | 801 °C |
Structure of Sodium chloride – NaCl
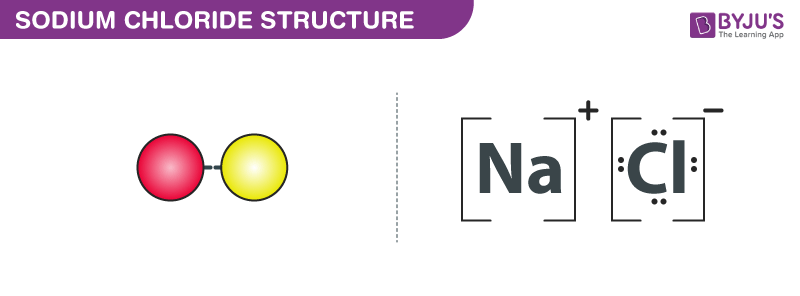
Structure of Sodium Chloride
Uses of Sodium chloride (NaCl)
- It is used in medicine – Saline solution in nasal spray
- It is used in fire extinguishers
- It is used in cleansers like shampoo, and toothpaste
- It is used in the soda ash industry to produce sodium carbonate through the Solvay process
- It is used in the paper industry, textile industry and in the construction of roads
- It is used in water softening
Recommended Videos

Frequently Asked Questions
What is sodium chloride used for?
The chemical name for common salt is sodium chloride. Sodium is an electrolyte that controls how much water the body has in it. Sodium also plays a part in muscle contractions and nerve impulses. Sodium chloride is used to treat or avoid dehydration, excessive sweating or other causes of sodium loss.
Is sodium chloride acidic or basic?
How to make 1M and 2M solutions of NaCl?
Fill a flask with 1litres of water, measure how much sodium chloride you need, add it to the water and shake until it is dissolved. Add 58.44 grams of salt for a 1M solution and add 116.88 grams and so on to make a 2M solution.
Is NaCl crystalline or amorphous solid?
It is a crystalline solid which have an fcc structure.
How many Na+ and Cl- ions are present in a unit cell of NaCl?
There will be 4 ions of each Na+ and Cl-.
Also, Read:
Sodium Borohydride
Titration of Hydrochloric Acid against Standard Sodium Carbonate
Titration of Oxalic Acid against Sodium Hydroxide
Further Reading:
| Bleaching Powder and Sodium Hydroxide | Organic Uses Of Sodium And Potassium |
| Chemical Reactions | Chemical Formula Of Common Compounds |
Learn more about the structure and properties of NaCl from the expert faculties at BYJU’S.

Comments