What is Ammonia?
Ammonia is a colorless gas with a chemical formula NH3.
It consists of hydrogen and nitrogen. In its aqueous form, it is called ammonium hydroxide. This inorganic compound has a pungent smell. In its concentrated form, it is dangerous and caustic. The NH3 chemical name is ammonia.
Table of Contents
- Ammonia Structure
- Preparation of Ammonia
- Properties of Ammonia
- Uses of Ammonia
- Frequently Asked Questions – FAQs
Ammonia is lighter than air with a density of 0.769 kg/m3 at STP. It is widely used as a fertilizer. It is also used in the manufacturing of explosives such as nitrocellulose and TNT. Also, it is used in the production of soda ash and in the Ostwald process to get nitric acid.
With the formula NH3, ammonia is a nitrogen and hydrogen inorganic chemical compound. Ammonia, the simplest pnictogen hydride and a stable binary hydride is a colourless gas with a strong, pungent odour. It contributes considerably to the nutritional demands of terrestrial creatures by serving as a precursor to 45% of the world’s food and fertilisers. Biologically, it is a common nitrogenous waste, especially among aquatic animals. Fertilizers in a variety of compositions, including urea and diammonium phosphate, are made with around 70% of the ammonia produced. Additionally, pure ammonia is sprayed straight onto the ground.
Ammonia Structure – NH3
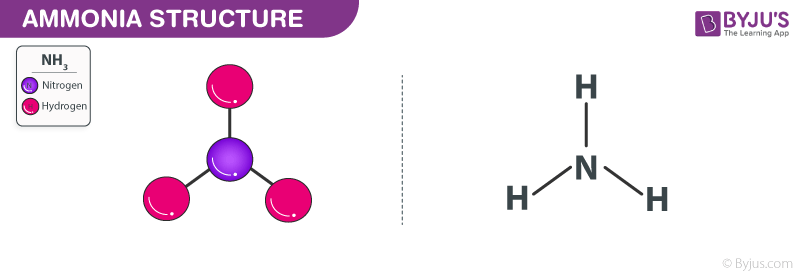
Ammonia Structure
Properties of Ammonia – NH3
| NH3 | Ammonia |
| Molecular Weight/ Molar Mass | 17.031 g/mol |
| Density | 0.73 kg/m³ |
| Boiling Point | -33.34 °C |
| Melting Point | −77.73 °C |
Ammonia is known to behave as a weak base since it combines with many acids to form salts. For example, when it is reacted with hydrochloric acid, ammonia is converted into ammonium chloride. All the salts that are produced from such acid-base reactions are known to contain the ammonium cation, denoted by NH4+. It is interesting to note that ammonia also exhibits weak acidic qualities and can, therefore, be regarded as an amphoteric compound. The acidic qualities of ammonia enable it to form amides with some alkali metals and alkaline earth metals. An example of such a reaction can be observed when lithium is exposed to liquid ammonia, triggering the formation of lithium amide (a chemical compound with the formula LiNH2).
It can also be noted that the NH3 molecule undergoes self dissociation when dissolved in water. The molecular autoionization of the ammonia molecule results in the formation of its conjugate base (NH2–) and its conjugate acid (NH4+). The structure of the ammonium cation is illustrated below.
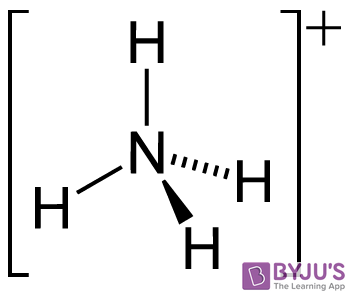
This autoionization process can be represented by the following equilibrium reaction:
2NH3 ⇌ NH2– + NH4+
Since ammonia generally functions as a relatively weak base, it can be used for buffering purposes (for the control of pH changes).
Preparation of Ammonia – NH3
Ammonia is easily made in the laboratory by heating an ammonium salt, such as ammonium chloride NH4Cl with a strong alkali, such as sodium hydroxide or calcium hydroxide.
2NH4Cl + Ca(OH)2 → CaCl2 + 2H2O + 2NH3(g)
The gas may also be made by warming concentrated ammonium hydroxide.
The principal commercial method of production of ammonia is the Haber Process, the direct combination of nitrogen and hydrogen under high pressure in the presence of a catalyst.
NH3 Uses (Ammonia)
- It is used as fertilizers as it increases the yield of crops
- It is used in the household as a cleaner – NH3 is mixed with water to clean stainless steel and glass
- It is used in food products as an antimicrobial agent
- It is used in the fermentation industry
- It is used as a refrigerant
- It is used as a pH adjuster in the fermentation process
- It is used to neutralize pollutant like nitrogen oxides emitted from diesel engines
- It is used as a fuel for rocket engines
- It is used in textile industries
- It is used in the manufacture of synthetic fibre like rayon and nylon
Solved Example
Consider the reaction N2(g)+3H2(g) –> 2NH3(g), The equality relationship between d[NH3]/dt and −d[H2]/dt is
Solution:
Therefore,
Frequently Asked Questions – FAQs
What is the chemical name of NH3?
The chemical name of NH3 is ammonia. It is also known as trihydridonitrogen and nitrogen trihydride. This compound is known to be the simplest pnictogen hydride.
What are the uses of ammonia?
One of the most important applications of ammonia is its use in the agriculture industry as a fertilizer. Ammonia, in its anhydrous form (or sometimes in aqueous solutions or in the form of ionic salts), is often mixed with agricultural soils in order to increase the nitrogen content in the soil and, therefore, the fertility of the soil. This is often accompanied by higher crop yields and better crop quality. This compound is also used in the synthesis of many important compounds such as hydrazine and hydrogen cyanide.
How is ammonia produced?
Up till the early 1900s, ammonia was mostly produced via the dry distillation of animal waste products along with certain vegetable waste that was rich in nitrogen. The distillation of these waste products resulted in the reduction of nitrites and nitrous acids along with hydrogen. Eventually, ammonia was obtained as a product. Today, ammonia is produced industrially via the Haber-Bosch process, which involves a reaction in the gaseous phase between molecular nitrogen and molecular hydrogen. It is important to note that this reaction takes place at relatively high temperatures and high pressure (in the order of 450 degrees celsius and over 10000 kilopascals).
Does liquid ammonia act as a solvent?
Liquid ammonia is the most commonly studied, and best known, non-aqueous ionizing solvent. The most notable property of this compound is its ability to dissolve alkali metals to form strongly coloured, electrically conductive solutions that contain solved electrons. Besides these notable solutions, much of the chemistry of liquid ammonia can be described with the help of aqueous solutions by comparison with similar reactions.
Where can ammonia be found naturally?
Ammonia is known to naturally occur in many parts of the environment such as the soil, the air, and in vegetation. It can also be noted that the human body naturally creates ammonia while breaking down protein-containing food items into amino acids. This ammonia is then converted into urea. It is important to note that ammonia and, by extension, the ammonium ion are important components of many vital metabolic processes in human beings.
What are the side effects associated with the inhalation of ammonia?
When large concentrations of ammonia are inhaled, the most common symptoms that arise include an immediate burning of the throat, nose, and respiratory tract. Eventually, this can lead to respiratory distress or respiratory failure. If the concentration of ammonia in the atmosphere is low, the common side effects are throat irritation and nose irritation.
Also, Read:
| Urea | Carbohydrates |
| Glucose | Phenol |
To know more about the structure, structure, and properties of NH3 from the expert faculties at BYJU’S register now.


It is very helpful for my chemistry preperation
My name is Somesh I am 15 years old and I learn all subjects in byjus thanks