What is Ester?
An ester is a chemical compound derived from an acid (organic or inorganic) in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group. To put it in simple terms, esters are a group of chemical compounds which are formed by bonding of an alcohol group with a group of organic acids, by losing water molecules.
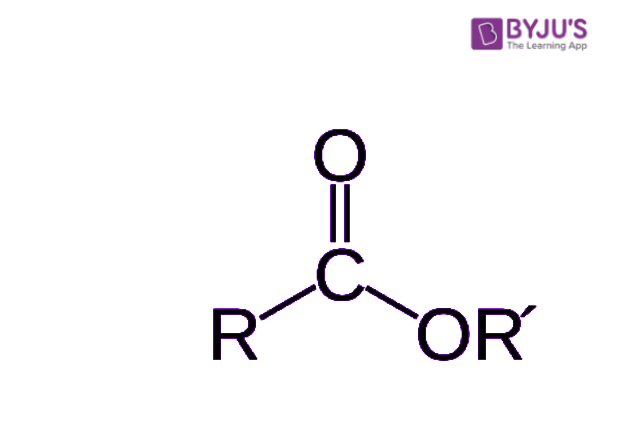
Table of Contents
Esters are also usually derived from carboxylic acids. It may also be obtained by reaction of acid anhydride or acid halides with alcohols or by the reaction of salts of carboxylic acids with alkyl halides.
What is Esterification?
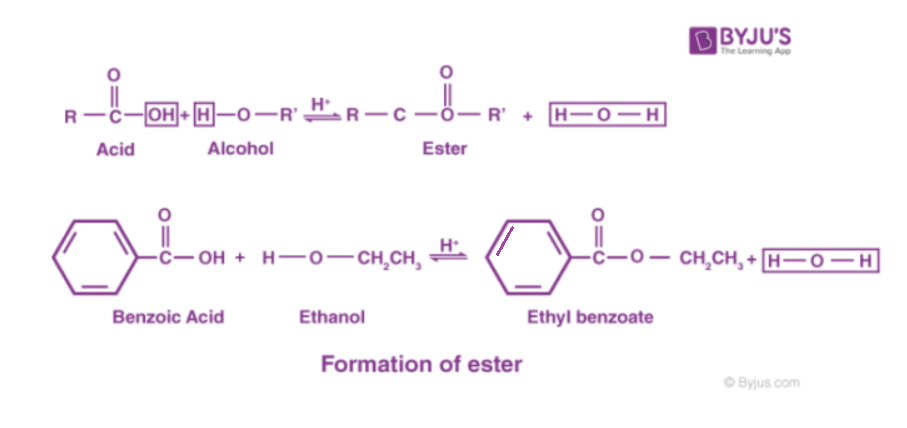
Esterification is a process or a general name for a chemical reaction, in which two reactants (alcohol and an acid) form an ester as the reaction product. The formula for carboxylic acid esters is RCOOR’ (where R and R’ are any organic combining groups) that are prepared by the reaction of alcohols and carboxylic acids in the presence of hydrochloric acid or sulphuric acid as catalyst.
To give you an example of an ester, we can talk about ethyl ethanoate. Here, the hydrogen in the -COOH group is replaced by an ethyl group. You can find the ethyl ethanoate formula below:
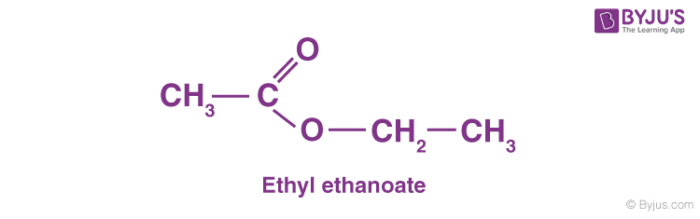
In this case, the ester is usually named in the opposite way around from the way the formula is written. In this case, the “ethanoate” part comes from ethanoic acid and the “ethyl” part comes from the ethyl group at the end.
Structure of Ester
Esters feature a carbon-to-oxygen double bond that is also singly bonded to a second oxygen atom. The oxygen atom is further connected to an aryl or an alkyl group. They come in all shapes and sizes. They can have a form as small as that of allyl hexanoate like pineapple odour or as large as a long-chain triglyceride, like soybean oil.
Ethyl Acetate
Methyl Butyrate
Interestingly, esters can also be split back to alcohols and carboxylic acids by the action of water, dilute acid or dilute alkali. This process is known as ester hydrolysis.
Uses of Esters
It is a sweet-smelling substance. Some of them are used as food flavourings and other esters are used as fragrances or perfumes. Apart from that, they can be turned into polymers dubbed as polyesters which can be used to make cans or plastic bottles.
Here are some other users of esters:
-
-
-
- Esters that have fragrant odours are used as a constituent of perfumes, essential oils, food flavourings, cosmetics, etc
- It is used as an organic solvent
- Natural esters are found in pheromones.
- Naturally occurring fats and oils are fatty acid esters of glycerol.
- Nitrate esters, such as nitroglycerin, are used as explosive materials.
- Polyesters can be further converted into fibres to make clothing.
- It is used to make surfactants E.g. soap, and detergents.
-
-
Also, Read: Transesterification
Frequently Asked Questions – FAQs
What is ester used for?
In synthetic flavours, perfumes, and cosmetics, these and other toxic esters with distinctive odours are used. Some volatile esters are used as solvents for coatings, paints and varnishes; significant amounts of ethyl acetate and butyl acetate are manufactured commercially for this purpose.
What is an ester in chemistry?
A compound or a functional group derived from alcohol condensation and acid with simultaneous water loss. Carboxylic ester (also referred to as carboxylate ester; also simply called an ester), derived from carboxylic acid, is the most common form of ester.
Why do esters smell?
Esters smell sweet because of the feeble intermolecular forces they show. This encourages ester molecules to penetrate and hit the nose in the gas phase. To share in hydrogen bonding, there are not highly positively polarised hydrogens in esters. Remember ethyl butyrate, for instance, which smells like pineapple.
How is an ester formed?
The condensation reaction between an alcohol and a carboxylic acid produces esters. This is referred to as esterification. Two molecules combine and create a larger molecule in a condensation reaction thus removing a tiny molecule. This small molecule, during esterification, is water.
What is the functional group of ether?
Ethers are a class of organic compounds containing an ether group, an atom of oxygen bound to two classes of alkyl or aryl. They have the general R-O-R’ formula, where the alkyl or aryl groups are denoted by R and R’.
Read more:
For more details on this topic or learn about more chemistry terms, you can download BYJU’S – The Learning App.


Comments