What is Carboxylic Acid?
A Carboxylic Acid is an organic compound containing a carboxyl functional group. They occur widely in nature and are also synthetically manufactured by humans. Upon deprotonation, carboxylic acids yield a carboxylate anion with the general formula R-COO–, which can form a variety of useful salts such as soaps.
Table of Content
Definition
The carboxylic acids are the most important functional group that present C=O. This type of organic compounds can be obtained by different routes, some carboxylic acids, such as citric acid, lactic acid or fumaric acid are produced by fermentation, most of these types of carboxylic acids are applied in the food industry.
Carboxylic Acid Structure
The general formula of a carboxylic acid is R-COOH, where COOH refers to the carboxyl group, and R refers to the rest of the molecule to which this group is attached. In this carboxyl group, there exists a carbon which shares a double bond with an oxygen atom and a single bond with a hydroxyl group.
A carboxylic acid’s general formula is R-COOH, where COOH denotes the carboxyl group and R denotes the remainder of the molecule to which this group is linked. There is a carbon in this carboxyl group that has a double connection with an oxygen atom and a single bond with a hydroxyl group.
The first four carboxylic acids derived from alkanes are methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH), and butanoic acid (C3H7COOH).
The general structure of a carboxylic acid is illustrated below.
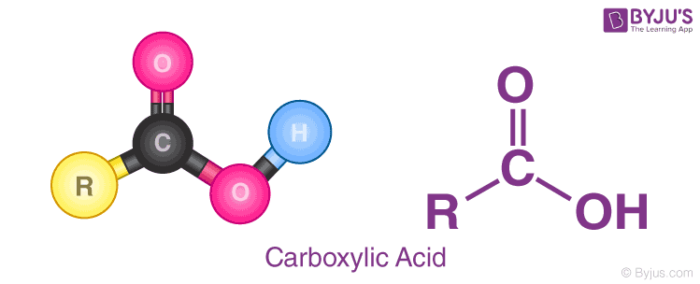
From the illustration provided above, it can be observed that a carboxylic acid contains a hydroxyl group attached to a carbonyl carbon. Due to the electronegativity of the oxygen atom, this functional group can undergo ionization and discharge a proton.
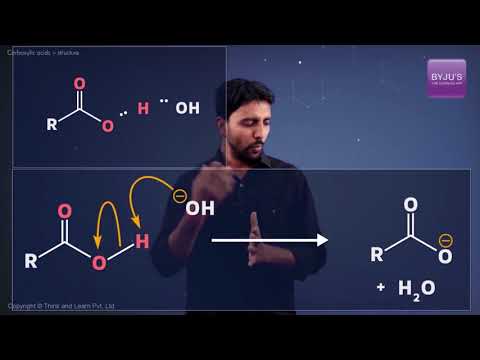
The carboxylate ion, produced from the removal of a proton from the carboxyl group, is stabilized by the presence of two oxygen atoms (through which the negative charge can move). Some common examples of carboxylic acids include acetic acid (a component of vinegar) and Formic acid.
The acidity of the carboxylic acid is explained in the video.
Nomenclature of Carboxylic Acids
Generally, these organic compounds are referred to by their trivial names, which contain the suffix “-ic acid”. An example of a trivial name for a carboxylic acid is acetic acid (CH3COOH). In the IUPAC nomenclature of these compounds, the suffix “-oic acid” is assigned.
The guidelines that must be followed in the IUPAC nomenclature of carboxylic acids are listed below.
- The suffix “e” in the name of the corresponding alkane is replaced with “oic acid”.
- When the aliphatic chain contains only one carboxyl group, the carboxylic carbon is always numbered one. For example, CH3COOH is named as ethanoic acid.
- When the aliphatic chain contains more than one carboxyl group, the total number of carbon atoms is counted and the number of carboxyl groups is represented by Greek numeral prefixes such as “di-”, “tri-“, etc.
- A carboxylic acid is named by adding these prefixes and suffixes to the parent alkyl chain. Arabic numerals are used for indicating the positions of the carboxyl group.
- The name “carboxylic acid” or “carboxy” can also be assigned for a carboxyl substituent on a carbon chain. An example of such nomenclature is the name 2-carboxyfuran for the compound 2-Furoic acid.
Carboxylic Acid Examples
Some examples describing the nomenclature of carboxylic acids as per IUPAC guidelines are provided below.
| Trivial Name and Formula | IUPAC Name of the Carboxylic Acid |
| Formic acid, H-COOH | Methanoic acid |
| Crotonic acid, CH3CH=CH-COOH | But-2-enoic acid |
| Carbonic acid, OH-COOH | Carbonic acid |
| Butyric acid, CH3(CH2)2COOH | Butanoic acid |
Properties of Carboxylic Acids
Most of the properties of carboxylic acids are a result of the presence of the carboxyl group. Some physical and chemical properties of these compounds are discussed in this subsection.
1. Physical Properties of Carboxylic Acids
- Carboxylic acid molecules are polar due to the presence of two electronegative oxygen atoms.
- They also participate in hydrogen bonding due to the presence of the carbonyl group (C=O) and the hydroxyl group.
- When placed in nonpolar solvents, these compounds form dimers via hydrogen bonding between the hydroxyl group of one carboxylic acid and the carbonyl group of the other.

- The solubility of compounds containing the carboxyl functional group in water depends on the size of the compound. The smaller the compound (the shorter the R group), the higher the solubility.
- The boiling point of a carboxylic acid is generally higher than that of water.
- These compounds have the ability to donate protons and are therefore Bronsted-Lowry acids.
- They generally have a strong sour smell. However, their esters have pleasant odours and are therefore used in perfumes.
2. Chemical Properties of Carboxylic Acids
- The α-carbon belonging to a carboxylic acid can easily be halogenated via the Hell-Volhard-Zelinsky reaction.
- These compounds can be converted into amines using the Schmidt reaction.
- A carboxylic acid can be reduced to an alcohol by treating it with hydrogen to cause a hydrogenation reaction.
- Upon reaction with alcohols, these compounds yield esters.
Uses of Carboxylic Acids
- Fatty acids that are essential to human beings are made up of carboxylic acids. Examples include omega-6 and omega-3 fatty acids.
- Higher fatty acids are also used in the manufacture of soaps.
- The production of soft drinks and many other food products involves the use of many carboxylic acids.
- The manufacture of rubber involves the use of acetic acid as a coagulant.
- Hexanedioic acid is used in the manufacture of nylon-6,6.
- Carboxylic acids have numerous applications in the rubber, textile, and leather industries.
- Ethylenediaminetetraacetic acid is a widely used chelating agent.
- The synthesis of many drugs involves the use of these compounds. Therefore, carboxylic acids are very important in pharmaceuticals.
- The production of many polymers involves the use of compounds containing the carboxyl functional group.
Frequently Asked Questions – FAQs
What Is carboxylic acid used for?
In the manufacture of polymers, biopolymers, coatings, adhesives, and prescription products, carboxylic acids and their derivatives are used. They can also be used as solvents, antimicrobials, food additives, and flavourings.
What are examples of carboxylic acids?
Carboxylic acids are hydrocarbon compounds in which a carboxyl group has substituted one or more of the hydrogen atoms in the hydrocarbon. Methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH), and butanoic acid (C3H7COOH) are the first four carboxyl acids derived from alkanes.
What foods contain carboxylic acids?
In plants and animals, certain carboxylic acids exist naturally. There is citric acid in citrus fruits, such as oranges and lemons. A large carboxylic acid with three ionizable hydrogen atoms is citric acid. It is present in citrus fruits and provides them with a sour or tart taste.
Why are carboxylic acids acidic?
The carboxylic acids are acidic because of the hydrogen in the -COOH group, using the idea of an acid as a “substance that donates protons (hydrogen ions) to other things.” A hydrogen ion is moved from the -COOH group onto a water molecule in a water solution.
Are carboxylic acids strong or weak?
Carboxylic acids are defined as weak acids, meaning that in a neutral aqueous solution, they do not fully dissociate to create H+ cations. Hydrogen bonds are formed between the individual molecules of the acid and water molecules. That’s why They partially ionise to give H+ and RCOO−. Therefore they are called weak acids.
Thus, the general formula, structure, nomenclature, properties, and uses of carboxylic acids are briefly discussed. To learn more about these compounds and other types of organic compounds, such as aldehydes and ketones, register with BYJU’S and download the mobile application on your smartphone.


Comments