What are Aldehydes and ketones?
Aldehydes and ketones incorporate a carbonyl functional group, C=O. These are organic compounds with structures -CHO and RC(=O)R’, where R and R’ represent carbon-containing substituents respectively.
Table of contents
- What Are Aldehydes?
- What Are Ketones?
- Recommended Videos
- Occurrence Of Ketones And Aldehydes
- Preparation Of Ketones And Aldehydes
- Uses Of Aldehydes And Ketones
- FAQs
What are Aldehydes?
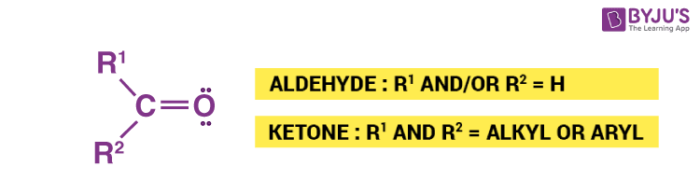
In aldehydes, the carbonyl group has one hydrogen atom attached to it together with either a 2nd hydrogen atom or a hydrogen group which may be an alkyl group or one containing a benzene ring.
Example:
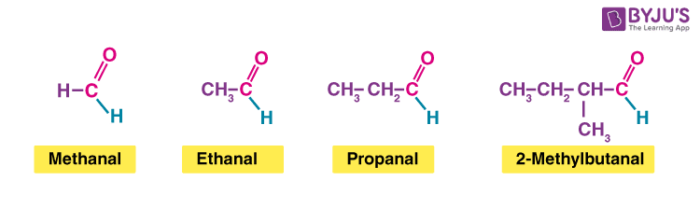
One can notice that all these have the exact same end to the molecule. The only difference is the complexity of the other attached group.
What are Ketones?
In ketones, the carbonyl group has 2 hydrocarbon groups attached to it. These can be either the ones containing benzene rings or alkyl groups. Ketone does not have a hydrogen atom attached to the carbonyl group.
Example:
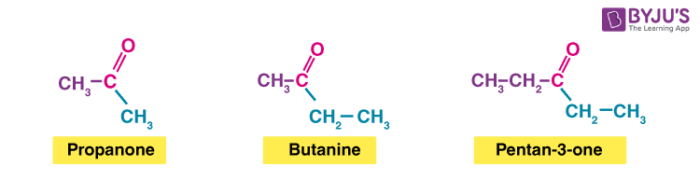
Propane is generally written as CH3COCH3. In pentanone, the carbonyl group could be in the middle of the chain or next to the end – giving either pentan-3-one or pentan-2-one.
Recommended Videos

Aldehydes and Ketones are often called as methanoyl or formyl group. The carbon atom of this group has 2 remaining bonds that might be occupied by aryl or alkyl or substituents. If neither of these substituents is hydrogen, the compound is a Ketone. If at least one is hydrogen, the compound is an Aldehyde.
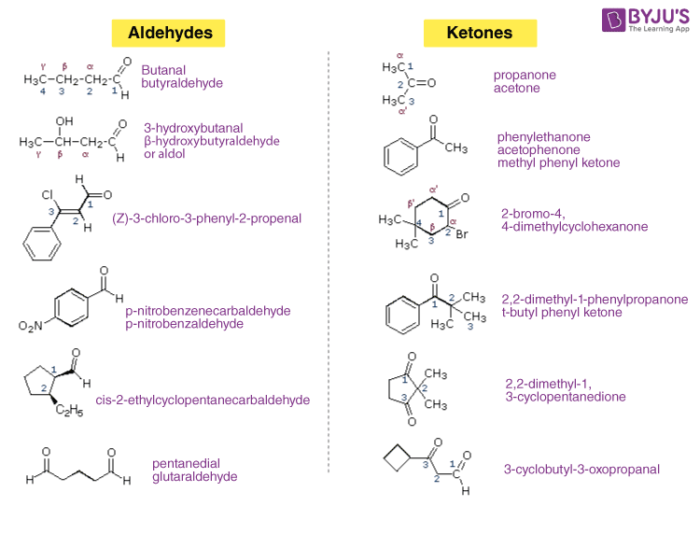
Occurrence of Ketones and Aldehydes
Combined with other functional group aldehydes and ketone are widespread in nature. Compounds such as cinnamaldehyde (cinnamon bark), vanillin (vanilla bean), Citra (lemongrass), helminthosporal (a fungal toxin), carvone (spearmint and caraway), camphor (camphor trees) are found chiefly in microorganisms or plants. Whereas, compounds such as muscone (musk deer), testosterone (male sex hormone), progesterone (female sex hormone), cortisone (adrenal hormone) have animal and human origin.
Preparation of Ketones and Aldehydes
Aldehydes and Ketones are obtained from products of many reactions. Some reactions for the synthetic preparation of Aldehyde and Ketone is mention below.
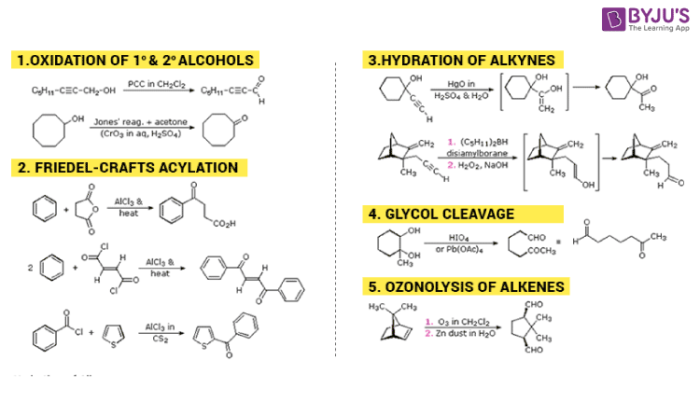
Stay tuned with BYJU’S to learn more about different types of aldehydes and ketones, their physical and chemical properties.
Uses of Aldehydes and Ketones
Formaldehyde is the simplest aldehyde whereas acetone is the smallest ketone. There are a number of aldehydes and ketones which find application due to their chemical properties. A few uses of Aldehydes and Ketones are listed below.
1. Uses of Aldehydes
- Formaldehyde is a gas. With 40% solution in water, it forms Formalin which is used in preserving biological specimens.
- Formaldehyde is used in embalming, tanning, preparing glues and polymeric products, as germicides, insecticides, and fungicides for plants. It is also used in drug testing and photography.
- When reacted with phenol, formaldehyde forms Bakelite, which is used in plastics, coatings, and adhesives.
- Acetaldehyde is largely used for the production of acetic acid and pyridine derivatives.
- Benzaldehyde is used in perfumes, cosmetic products, and dyes. It is added to provide almond flavour to food products and also used as a bee repellent.
2. Uses of Ketones
- The most common ketone is acetone which is an excellent solvent for a number of plastics and synthetic fibres.
- In the household, acetone is used as a nail paint remover and paint thinner.
- In medicine, it is used in chemical peeling and for acne treatments.
- Methyl ethyl ketone (MEK), chemically butanone, is a common solvent. It is used in the production of textiles, varnishes, plastics, paint remover, paraffin wax, etc.
- MEK is also used as a welding agent for plastics due to its dissolving properties.
- Cyclohexanone is another important ketone which is primarily used in the production of nylon.
Acetaldehyde and Acetone can be distinguished by
Tollens’ reagent is silver ammonia complex or ammoniacal AgNO3 solution.
It oxidizes acetaldehyde to acetic acid. Silver ions are reduced to silver metal. This gives silver mirror. Such silver mirror is not obtained with acetone. Hence, Tollens’ reagent is used to distinguish acetaldehyde and acetone.
Molisch, Schiffs, Iodoform reacts similar with aldehydes and ketones. But Tollen’s reagents gives silver mirror only to aldehyde not to ketone.
Frequently Asked Questions
How are Aldehydes and Ketones Different?
The carbonyl carbon of an aldehyde has a hydrogen atom attached to it whereas that of a ketone is attached to two alkyl or aryl groups. The C-H bond in aldehydes makes them easily oxidizable (they are strong reducing agents).
Why are Aldehydes more Reactive towards Nucleophilic Substitutions than Ketones?
The two alkyl/aryl groups in ketones offer steric hindrance during substitution reactions. Since the hydrogen atom is relatively small, it barely offers any steric hindrance. This is the primary reason why aldehydes are more susceptible to nucleophilic substitutions. Also, the partially positive charge on the carbonyl carbon is stabilized by the two R groups in ketones.
Why are the Boiling Points of Ketones Higher than those of Aldehydes?
The presence of two electron-donating R-groups in ketones makes them more polar than aldehydes. The dipole moments that arise from this polarity account for the higher boiling points of ketones.
What are the uses of Ketones?
Ketones are used as excellent solvent in industry, acetone is used as a nail paint remover and paint thinner. They are also used in medicine , textiles, varnishes, plastics, paint remover, paraffin wax etc.
What are the uses of aldehydes?
The 40% solution of formaldehyde forms Formalin which is used in preserving biological specimens. It is also used in embalming, tanning, preparing glues and polymeric products, as germicides, insecticides, fungicides for plants, drug testing and photography etc. Acetaldehyde used in the production of acetic acid and pyridine derivatives.Benzaldehyde is used in perfumes, cosmetic products, and dyes. It is added to provide almond flavour to food products and also used as a bee repellent .


Comments