What is Phenolphthalein?
Phenolphthalein is an organic compound which is used medicinally as a cathartic. It has a chemical formula C20H14O4. It is written as “phph” or HIn”. It is used in acid-base titration as an indicator. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. It is either yellowish-white to pale orange or white fine crystalline powder and in its liquid form it appears as a colourless till PH 8.5 and above that it appears as pink to deep red. It does not have taste and smell. This chemical compound is widely used as PH indicator and laboratory agent. Under acidic conditions when phthalic anhydride is condensed with two equivalents of phenol, Phenolphthalein is synthesized. It is a weak acid and belong to class of dyes called Pthalein dyes
Properties of Phenolphthalein – C20H14O4
| C20H14O4 | Phenolphthalein |
| Molecular Weight/ Molar Mass | 318.32 g/mol |
| Density | 1.277 g/cm3 |
| Appears | White powder |
| Melting Point | 258–263 °C |
Phenolphthalein Structure – C20H14O4
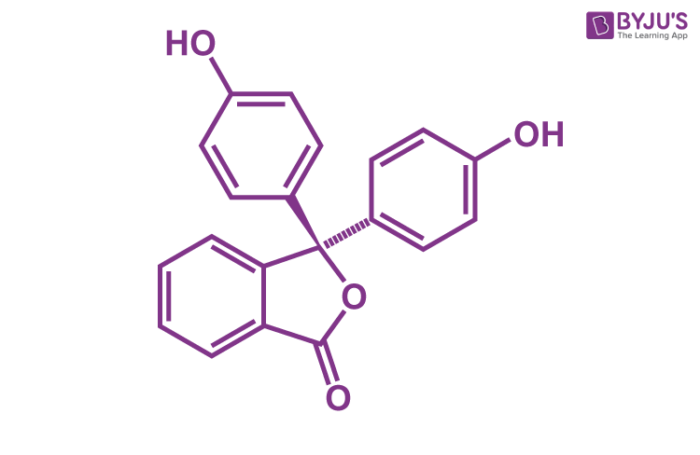
C20H14O4 Uses (Phenolphthalein)
- It is used in acid base titrations as an indicator
- It is used in Kastle-Meyer test
- It was before used as a laxative
Also, Read:
| Benzoic Acid | Acetic Acid |
| Sodium Hydroxide | Sulfuric Acid |
Frequently Asked Questions
What are the uses of Phenolphthalein?
One popular application of phenolphthalein is as an indicator in acid-base titrations. Phenolphthalein is somewhat water-soluble, and is typically dissolved in alcohols for experimental use. It is a weak acid which, in solutions, can lose H+ ions. The molecule of phenolphthalein is colourless, and the ion of phenolphthalein is purple.
Is phenolphthalein acidic or basic?
Phenolphthalein, an organic compound with the chemical formula C20H14O4, is a weak acid which can be used as an indicator for acid-base titrations. In acidic solutions, the compound is colourless. It is pinkish in simple solutions (with the transition occurring around pH 9).
How does phenolphthalein function as an indicator?
Phenolphthalein is a colourless, weak acid that is widely used as an indicator in titration experiments to indicate the endpoint of the titration. The endpoint is indicated by the formation of a pink colour since this compound dissociates to form pink anions when dissolved in water.
What is the Colour of Phenolphthalein?
Phenolphthalein is a white or colourless chemical compound.
Subscribe to BYJU’S to learn more about the structure of C20H14O4 from the expert faculties.

Q1. Why acid turns phenolphthalein to pink?
Q2. Why bases turn methyl orange to red ?
A1. Phenolphthalein is a weak acid and is colorless in solution although its ion is pink. If hydrogen ions (H+, as found in an acid) were added to the pink solution, the equilibrium would switch, and the solution would be colorless. Adding hydroxide ions (OH-, as found in bases) will change the phenolphthalein into its ion and turn the solution pink.
A2. Methyl orange acts as an indicator, to help us identify the acidic and basic medium by undergoing colour change. As the acidity of the solution decreases, the intensity of red colour decreases, the red colour changes to orange and orange turns yellow as the pH increases and acidity decreases.
in what form does it exist in acidic and alkaline forms?
The change in pH converts one tautomeric form into other and thus, the colour change occurs. Phenolphthalein has benziod form in acidic medium and thus, it is colourless while it has quinonoid form in alkaline medium which has pink colour.