What is Salt in Chemistry?
Salt is an ionic compound that contains a cation (base) and an anion (acid).
It is present in large quantities in seawater, where it is the main mineral constituent. Salt is essential for animal life and saltiness is one of the basic human tastes. Salt is an ionic compound that has a cation other than H+ and an anion other than OH– and is obtained along with water in the neutralization reaction between acids and bases.
Eg:- NaCl, CuCl2 etc.
Acid + Base → Salt + water
Sodium chloride is one of the best-known salt. One salt is known to almost everyone because of its widespread use in every day.
Recommended Videos

Types of Salt
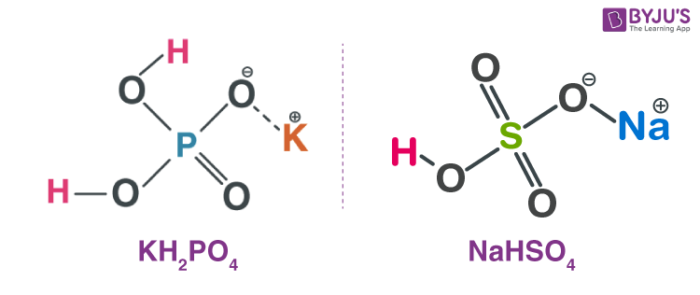
1. Acidic salt – The salt formed by partial neutralization of a diprotic or a polyprotic acid is known as an acidic salt. These salts have ionizable H+ ion along with another cation. Mostly the ionizable H+ is a part of the anion. Some acid salts are used in baking.
For eg:- NaHSO4, KH2PO4 etc.
H2SO4 + NaOH → NaHSO4 + H2O
2. Basic or Alkali Salt – The salt formed by the partial neutralization of a strong base by a weak acid is known as a basic salt. They hydrolyze to form a basic solution. It is because when hydrolysis of basic salt takes place, the conjugate base of the weak acid is formed in the solution.
For eg:- White lead (2PbCO3·Pb(OH)2).
3. Double salt – The salts that contain more than one cation or anion are known as double salt. They are obtained by the combination of two different salts crystallized in the same ionic lattice.
For eg:- Potassium sodium tartrate (KNaC4H4O6.4H2O) also known as Rochelle salt.
4. Mixed Salts – The salt that consists of a fixed proportion of two salts, often sharing either a common cation or common anion is known as mixed salt.
For e.g. :- CaOCl2
Properties of Salt
The compound’s sodium chloride has very different properties from the elements sodium and chlorine.
- Saltwater contains ions and is a fairly good conductor of electricity.
- This electrostatic force of attraction holds the ions together and a chemical bond is said to form between them.
Hydrolysis of a Salt
Hydrolysis of salt refers to the reaction of salt with water. It is the reverse of a neutralization reaction. In this reaction, when salt undergoes reaction with water, the constituent acid and base are formed as products. In hydrolysis, the salt dissociates to form ions, completely or partially depending upon the solubility product of that salt.
For a detailed discussion on acids, bases and salts and the reactions salts undergo like Hydrolysis of salts, check out our app BYJU’S – The Learning App.

Thank you very much for this information.