What is Nitrous Oxide?
Nitrous oxide (N2O), also known as laughing gas, is commonly used as an anaesthetic and is therefore interesting for a variety of medical applications.
Nitrous oxide is an oxide of nitrogen with a chemical formula N2O. This organic compound is colourless and non-flammable at room temperature. It is also known as nitrous or laughing gas. Joseph Priestley was the first to identify nitrous oxide in the year 1772.
This compound is insoluble in water and works as a powerful oxidizer at higher temperatures. It has a slightly sweet odour and appears as a colourless gas. When inhaled in a small amount it causes mirth and euphoria. It is the world’s number one inhaled anaesthetic as it works as a quick pain reliever. It can cause a narcotic effect at higher concentrations and lead to death by asphyxia. The laughing gas formula is written as N2O.
Table of Contents
- Structure Of Nitrous Oxide
- Properties Of Nitrous Oxide
- Recommended Video Nitrous Oxide
- Preparation Of Nitrous Oxide
- Uses Of Nitrous Oxide
- Faqs
Nitrous Oxide Structure – N2O
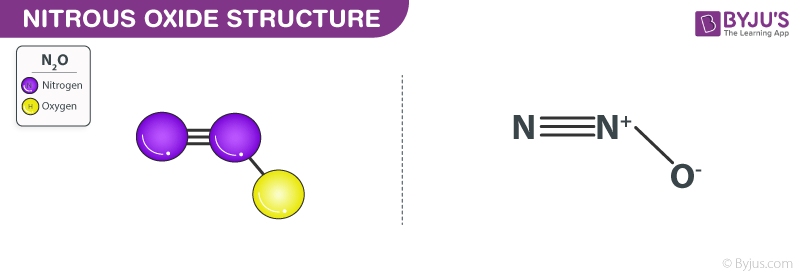
Structure of Nitrous oxide
Properties of Nitrous Oxide (N2O)
| N2O | Nitrous oxide |
| Molecular Weight/ Molar Mass | 44.013 g/mol |
| Density | 1.98 kg/m³ |
| Boiling Point | -88.48 °C |
| Melting Point | −90.86 °C |
Recommended Videos

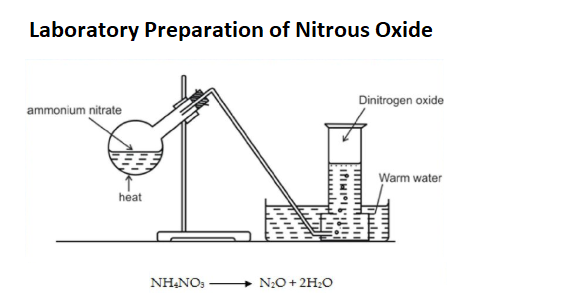
Preparation of Nitrous Oxide (N2O)
- Nitrous oxide (N2O) is a long-lived important greenhouse gas that is commonly known as “laughing gas” because of its use as an anaesthetic in medical procedures.
- Nitrous oxide is always prepared from the nitrate of ammonia. Some attention must be paid to the purity of the salt which should contain no hydrochlorate or ammonia.
- It is formed by adding pounded carbonate of ammonia to pure nitric acid, which is concentrated and may be previously diluted with half its bulk of water so long as there is effervescence and a small excess of the carbonate may be left at the end in the liquor.
- The solution is concentrated on its boiling point begins to rise above 250oC and a drop of it becomes solid on a cool glass plate.
- To obtain nitrous oxide a quantity of this salt is introduced into a retort and heated by activated charcoal, the diffused heat of which is more suitable than the heat of the lamp.
- At a temperature not under 340oC, the salt boils and begins to undergo decomposition being resolved into nitrous oxide and water.
- Nitrous oxide should be collected in a gasometer or in a gas holder filled with water of temperature about 90o as cold water absorbs much of this gas.
- The whole salt undergoes the same decomposition and nothing whatever is left in the retort.
- Nitrous oxide is likewise produced when the salt called nitrogen sulphate of ammonia is thrown into acid and also when dissolved in dilute nitric acid but later processes do not afford the gas in the state of purity.
Uses of Nitrous Oxide (N2O)
- It is used in rocket motors as an oxidiser
- It is used as a food additive as an aerosol spray propellant
- It is used in the manufacturing of semiconductors
- It is used in the medical field as an analgesic and anaesthetic
- It is used as a flavouring ingredient
- It is used in car racing as a fuel additive
- It is used in dentistry
- It is used to manufacture chemicals
- It is used in surgery
Frequently Asked Questions – FAQs
What nitrous oxide is used for?
Nitrous oxide has significant medical uses for its anaesthetic and pain-reducing effects, particularly in surgery and dentistry. The colloquial name, invented by Humphry Davy, is due to the euphoric effects of inhaling it, a quality that has contributed to its therapeutic use as a dissociative anaesthetic.
How is nitrous oxide used in everyday life?
Colourless gas (N2O) is used in medical or dental surgery as an anaesthetic or analgesic. It is known as laughing gas because it gives rise to excitement. It is also used in the production of foods under pressure. It is also used in the processing of foods under strain.
What elements make up nitrous oxide?
Oxide with nitrous. Nitrous oxide (N2O), also known as dinitrogen monoxide, laughing gas or nitrous, is one of several nitrogen oxides, a colourless gas with a fun, sweet smell and taste that, when inhaled, induces insensitivity to pain accompanied by mild hysteria, often laughter.
How long does nitrous oxide last?
The sedation effect of nitrous oxide is experienced in minutes, and the effect wears off within minutes of stopping the gas. The effect of sedation takes from 30 seconds to three or four minutes to start anywhere.
What type of bond is nitrous oxide?
All gases are nitrogen and oxygen. Therefore, the most common type of bonding will be covalent. Electrons are exchanged in a covalent bond between two molecules. An ionic bond is when one molecule picks up an electron from the other, closer to the other molecule.
Also, Read:
| Citric Acid | Calcium Hydroxide |
| Carbonic Acid | Ethylene Glycol |
To know more about the importance, properties, and structure of N2O from the expert faculties at BYJU’S register now.

Comments