What are Reducing Agents?
A substance which loses electrons to other substances in a redox reaction and gets oxidised to a higher valency state is called a reducing agent.
A reducing agent is one of the reactants of an oxidation-reduction reaction which reduces the other reactant by giving out electrons to the reactant. If the reducing agent does not pass electrons to other substances in a reaction, then the reduction process cannot occur. For example, in the given reaction;
H2(g) + F2(g) → 2HF(g)
Hydrogen acts as a reducing agent because it donates its electrons to fluorine, which allows fluorine to be reduced.
Table of Contents
Recommended Videos

Characteristics of Reducing Agent
-
-
-
- Reducing agents tend to give away electrons. The metals of the s-block in the periodic table are said to be good reducing agents.
- The reducing agent after losing electrons gets oxidised and also causes the opposite reactant to get reduced by supplying electrons.
- Good reducing agents tend to consist of atoms with a low electronegativity (the ability of an atom or molecule to attract bonding electrons) and elements having relatively small ionisation energies serve as good reducing agents too.
- All the oxidation and reduction reactions involve the transfer of electrons.
- When some substance is oxidised, it is said to lose electrons and the substance which receives electrons is said to be reduced.
- If the substance has a strong tendency to lose electrons, then it is said to be a strong reducing agent (since it will reduce the other substances by donating electrons).
- Atoms with relatively large atomic radii tend to be better reductants.
-
-
Strong Vs Weak Reducing Agent
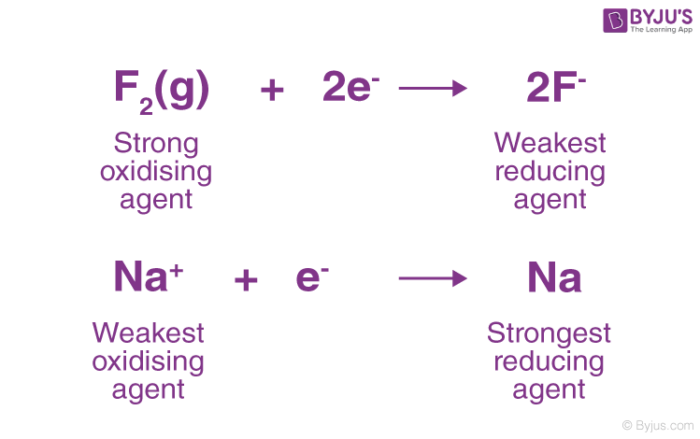
The stronger the reducing agent, the weaker is the corresponding oxidising agent. Fluorine gas is known to be a strong oxidising agent and whereas F- is said to be a weak reducing agent. We also know that – the weaker an acid the stronger is the conjugate base. Similarly, the weaker the oxidising agent, the more strong is the corresponding reducing agent, as shown in the figure below.
Reducing Agent Example
Some common reducing agents include metals such as Na, Fe, Zn, Al and non-metals such as C, S, and H2. Some compounds and also the Hydracids such as HCl, HI, HBr, and H2S behave as good reducing agents. A brief explanation of some reducing agents are given below-
-
-
-
- Lithium– Lithium is a chemical element with atomic number 3 and a symbol Li. It appears like a soft and silvery-white metal and belongs to the alkali metal group of the periodic table. It is said to be a strong reducing agent when placed in solutions.
- Iodides– The salts of Iodides are said to be mild reducing agents. They react with oxygen to give out iodine. These also possess various antioxidant properties.
- Reducing sugars– Reducing sugars are those which behave similarly to that the reducing agents because of the free ketone group or a free aldehyde group present. All monosaccharides, along with disaccharides, polysaccharides, and oligosaccharides are said to be reducing sugars.
-
-
Frequently Asked Questions – FAQs
Which is the strongest reducing agent?
Due to the smallest standard reduction potential, lithium is the strongest reducing agent.
Which is the weakest reducing agent?
The highest oxidising agent is the weakest reducing agent. Cu is the weakest reducing agent.
Why is hydrogen a good reducing agent?
When hydrogen gas is carried over warm metallic oxides of copper, lead, iron, etc., it removes oxygen from them and lowers them to their respective metal.
Why is iodine the best reducing agent?
Iodine is the least electronegative of all. So it will be easier for I− to lose an electron and act as a reducing agent.
Some other compounds of reducing agents include Carbon, Carbon monoxide, Ascorbic acid, Sulphur dioxide, Hydrogen, Oxalic acid, Phosphites, phosphorous acid, hypophosphites, etc.

Comments