What are Rubber products?
There are many rubber products which we come across in our daily life. Some common rubber-based objects that we encounter in our day-to-day lives include rubber gloves, rubber bands, and rubber footwear. Rubber items have the ability to recover their shapes after being stretched or distorted, which is the reason why rubber can be classified as an elastomer. Rubber is an elastic substance which can be obtained both naturally (natural rubber) or artificially (they can also be synthesized chemically in laboratories; synthetic rubber-like butyl rubber, neoprene, etc.)
Table of Contents
- Natural rubber
- Preparation of Natural rubber
- Synthetic rubber
- Preparation of Natural rubber
- Uses of rubber
- Frequently Asked Questions
Types of rubber
There are two primary types of rubber, namely natural rubber and synthetic rubber.
Natural rubber
These are the elastomers which are obtained naturally. Natural rubber is made up of solid particles suspended in a milky white liquid (called latex) that drips from the bark of certain tropical and subtropical trees. This latex rubber is mainly found in countries like Brazil, India, Indonesia, Malaysia, and Sri Lanka. It is made by the polymerization of isoprene (2 methyl-1, 3-butadiene) which has a chemical formula (C5H8) n and it is known as cis- 1, 4- polyisoprene. In simple words, we can say that they are made by loosely joining the monomers of isoprene (C5H8) in the form of a long tangled chain.
Preparation of Natural Rubber:
- Rubber tapping – The milky white liquid latex is collected from the rubber trees in a cup by making a slight V-cut on the tree bark. The collected latex is washed, filtered and reacted with acids to congeal the rubber particles.
- Mastication – The rubber obtained from the tapping process is still not ready to be used. When it is cold it is very brittle in nature and when warmed up it becomes very gluey. To remove the brittle nature and strong odour of the rubber, it is allowed to pass through the rollers and is pressed to make it softer and flexible to work. This process is repeated based on the properties that are required for the rubber. In this process, extra chemical ingredients are also added to enhance the properties of rubber.
- Calendering is a process which is mainly performed to provide shape to the rubber using rollers (after proper mixing of the chemical ingredients).
- The final product obtained is then extruded to make hollow tubes by passing them through specially designed holes in an extrusion machine.
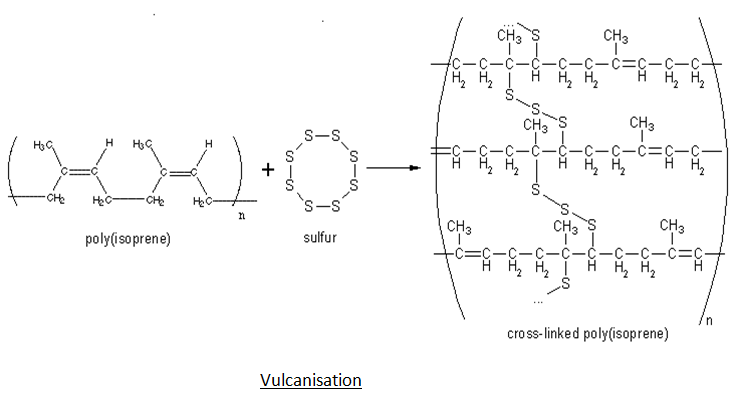
- Vulcanization – Performing all the steps listed above will not yield rubber that is strong or hard enough to be used in items like car tires and machinery. To enhance all these properties, sulphur is added to the rubber and it is heated at a temperature ranging from 373 K to 415 K. This process is known as vulcanization. The sulphur acts as a cross-linking agent and after vulcanization, rubber gets cross-linked and becomes hard.
Synthetic rubbers
Synthetic rubbers are produced from petroleum and natural gas. It is obtained by polymerization of 1, 3 – butadiene derivatives or by copolymerization of 1, 3 – butadiene along with an unsaturated monomer.
Preparation of synthetic rubbers:
Neoprene (Polychloroprene): –
The monomer of Neoprene is 2-chloro-1,3-butadiene, it is commonly known as chloroprene. Neoprene is a polymer of chloroprene, which is formed by joining together the monomers of chloroprene. Buna- N:-
Buna- N:-
It is a copolymer of 1, 3 – butadiene and acrylonitrile, it is formed in the presence of a peroxide catalyst.
Uses of Rubber
Rubber can be used for various rubber and across various platforms, a few of them are mentioned below;
- It is used for lining chutes, bins and industrial mixers. Because of its water-proof and resilient property, it can be made into a good insulator.
- In the clothing industry, it can be used as wetsuits and expandable clothes such as gym and cycling shorts etc.
- Rubbers are also used for flooring purposes it gives padding and prevents fatigue along with being waterproof and slip-resistant.
- In the automobile industry, its use can be witnessed in tires, padding in brakes, airbags, seats, roofs etc.
This article only provides a brief introduction to the preparation of rubber products. To know more about natural rubber products and how they are made, register with BYJU’S and download our app.
Frequently Asked Questions-FAQs
1. What is the difference between natural and synthetic rubber?
Natural rubber is polyisoprene in which monomer units are of isoprene i,e 2-methyl-1,3 butadiene. Natural rubber is obtained in the form of solid particles suspended in a milky white liquid (called latex) that drips from the bark of certain tropical and subtropical trees. Neoprene is a synthetic rubber. The monomer of Neoprene is 2-chloro-1,3-butadiene, it is commonly known as chloroprene.
2. What is vulcanisation rubber with an example?
Vulcanization is a chemical process in which the natural rubber is heated with sulphur, accelerator and activator at 373-415 K. Vulcanised rubber is better than natural rubber in the sense that it is more elastic, has less Water absorbing tendency and is more resistant to oxidation and towards organic solvent.
3. What is natural rubber used for?
Natural rubber is made up of solid particles suspended in a milky white liquid (called latex) that drips from the bark of certain tropical and subtropical trees. It is used in medical devices, surgical gloves, aircraft and car tires, pacifiers, clothes, toys, etc.
4. What are the disadvantages of natural rubber?
The disadvantages of natural rubbers are quite less resistant to attack by organic acid. It has little durability. When stretched to a greater extent, it suffers permanent deformation.
5. What are synthetic rubber examples?
Synthetic rubbers are produced from petroleum and natural gas. It is obtained by polymerization of 1, 3 – butadiene derivatives or by copolymerization of 1, 3 – butadiene along with an unsaturated monomer.


Extremely happy to read and collect information from Byjus website. This is because when I searched for a small information related to a particular topic, I got complete information related to that subject. I think Byjus (app and) website is a Knowledge and database think centre. It can also be called as Expert system. Thank you very much.